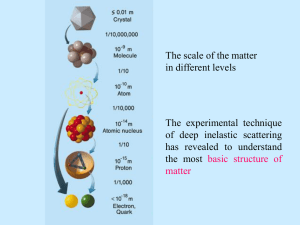
Scattering
advertisement
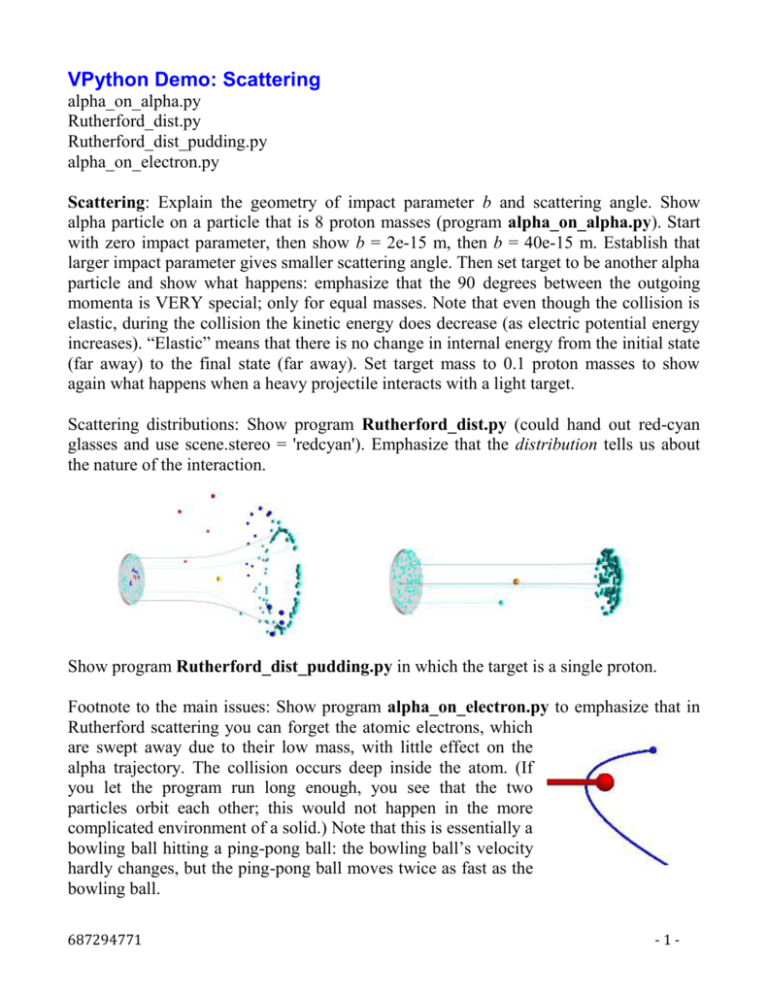
VPython Demo: Scattering alpha_on_alpha.py Rutherford_dist.py Rutherford_dist_pudding.py alpha_on_electron.py Scattering: Explain the geometry of impact parameter b and scattering angle. Show alpha particle on a particle that is 8 proton masses (program alpha_on_alpha.py). Start with zero impact parameter, then show b = 2e-15 m, then b = 40e-15 m. Establish that larger impact parameter gives smaller scattering angle. Then set target to be another alpha particle and show what happens: emphasize that the 90 degrees between the outgoing momenta is VERY special; only for equal masses. Note that even though the collision is elastic, during the collision the kinetic energy does decrease (as electric potential energy increases). “Elastic” means that there is no change in internal energy from the initial state (far away) to the final state (far away). Set target mass to 0.1 proton masses to show again what happens when a heavy projectile interacts with a light target. Scattering distributions: Show program Rutherford_dist.py (could hand out red-cyan glasses and use scene.stereo = 'redcyan'). Emphasize that the distribution tells us about the nature of the interaction. Show program Rutherford_dist_pudding.py in which the target is a single proton. Footnote to the main issues: Show program alpha_on_electron.py to emphasize that in Rutherford scattering you can forget the atomic electrons, which are swept away due to their low mass, with little effect on the alpha trajectory. The collision occurs deep inside the atom. (If you let the program run long enough, you see that the two particles orbit each other; this would not happen in the more complicated environment of a solid.) Note that this is essentially a bowling ball hitting a ping-pong ball: the bowling ball’s velocity hardly changes, but the ping-pong ball moves twice as fast as the bowling ball. 687294771 -1- Also stress difference between nuclear scale (10–14 m) and atomic scale (10–10 m). Show that we can ignore all but one nucleus in the scattering. At far left of blackboard draw a dot 0.5 mm in radius to represent one gold nucleus. Have students calculate how far away, to scale, (the next gold nucleus should be drawn. This is about 5 meters! (Factor of about 104). Draw another tiny dot far away. The essence of the Rutherford experiment is one alpha at a time colliding (electrically) with just one gold nucleus, and no electrons around. Even if the 79 protons were spread apart, they too would be nearly isolated. 687294771 -2-