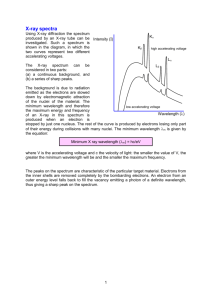
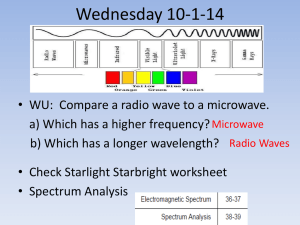
Emission Spectra - Juniata College
advertisement

Juniata College Science in Motion Emission Spectra “The Elements” Juniata College Science in Motion Juniata College Science in Motion Vocabulary Atom Element Spectrum (any of the following types of spectrum listed below) Electromagnetic spectrum (EM) Element Molecule Wavelength Juniata College Science in Motion Vocabulary All definitions are from http://dictionary.reference.com Atom A unit of matter, the smallest unit of an element, having all the characteristics of that element and consisting of a dense, central, positively charged nucleus surrounded by a system of electrons. The entire structure has an approximate diameter of 10-8 centimeter and characteristically remains undivided in chemical reactions except for limited removal, transfer, or exchange of certain electrons. This unit regarded as a source of nuclear energy. Element A substance that cannot be reduced to simpler substances by normal chemical means and that is composed of atoms having an identical number of protons in each nucleus. Any of more than 100 fundamental substances that consist of atoms of only one kind and that singly or in combination constitute all matter Each element emits a unique spectrum. These elements can be identified by their wavelengths in nanometers. http://hyperphysics.phy-astr.gsu.edu/hbase/vision/specol.html#c1 Emit to give off or send out (the process is called emission) Energy : the ability to do work; light is a form of energy Light Electromagnetic radiation that has a wavelength in the range from about 4,000 (violet) to about 7,700 (red) angstroms and may be perceived by the normal unaided human eye. Common term for electromagnetic radiation of any wavelength Juniata College Science in Motion The Spectrum of Visible Light The visible part of the spectrum may be further subdivided according to color, with red at the long wavelength end and violet at the short wavelength end, as illustrated (schematically) in the following figure. The visible spectrum http://csep10.phys.utk.edu/astr162/lect/light/spectrum.html http://www.nsta.org/main/news/stories/science_teacher.php?news_story_ID=48612&print=yes Juniata College Science in Motion Molecule: The smallest particle of a substance that retains the chemical and physical properties of the substance and is composed of two or more atoms; a group of like or different atoms held together by chemical forces. A molecule will emit a spectrum from the combination of elements that it possesses. (Physics) The smallest part of any substance which possesses the characteristic properties and qualities of that substance, and which can exist alone in a free state. (Chem.) A group of atoms so united and combined by chemical affinity that they form a complete, integrated whole, being the smallest portion of any particular compound that can exist in a free state; as, a molecule of water consists of two atoms of hydrogen and one of oxygen. Cf. Atom. Spectrum The distribution of a characteristic of a physical system or phenomenon, especially: The distribution of energy emitted by a radiant source, as by an incandescent body, arranged in order of wavelengths. The distribution of atomic or subatomic particles in a system, as in a magnetically resolved molecular beam, arranged in order of masses. The distribution of a characteristic of a physical system or phenomenon, especially the distribution of energy emitted by a radiant source arranged in order of wavelengths. The color image presented when white light is resolved into its constituent colors: red, orange, yellow, green, blue, indigo, violet. The plot of intensity as opposed to wavelength of light emitted or absorbed by a substance, usually characteristic of the substance and used in qualitative and quantitative analysis. The distribution of atomic or subatomic particles in a system, as in a magnetically resolved molecular beam, arranged in order of masses. a continuum of color formed when a beam of white light is dispersed (as by passage through a prism) so that its component wavelengths are arranged in order any of various continua that resemble a spectrum in consisting of an ordered arrangement by a particular characteristic (as frequency or energy ELECTROMAGNETIC SPECTRUM (2) : MASS SPECTRUM c : the representation (as a plot) of a spectrum. (a)The several colored and other rays of which light is composed, separated by the refraction of a prism or other means, and observed or studied either as spread out on a screen, by direct vision, by photography, or otherwise. (b) A luminous appearance, or an image seen after the eye has been exposed to an intense light or a strongly illuminated object. When the object is colored, the image appears of the complementary color, as a Juniata College Science in Motion green image seen after viewing a red wafer lying on white paper. Called also ocular spectrum. Absorption spectrum, the spectrum of light which has passed through a medium capable of absorbing a portion of the rays. It is characterized by dark spaces, bands, or lines. The electromagnetic spectrum, broken by a specific pattern of dark lines or bands, observed when radiation traverses a particular absorbing medium. The absorption pattern is unique and can be used to identify the material. Chemical spectrum, a spectrum of rays considered solely with reference to their chemical effects, as in photography. These, in the usual photogrophic methods, have their maximum influence at and beyond the violet rays, but are not limited to this region. Chromatic spectrum, the visible colored rays of the solar spectrum, exhibiting the seven principal colors in their order, and covering the central and larger portion of the space of the whole spectrum. Continous spectrum, a spectrum not broken by bands or lines, but having the colors shaded into each other continously, as that from an incandescent solid or liquid, or a gas under high pressure. Diffraction spectrum, a spectrum produced by diffraction, as by a grating. Emission Spectrum, an electromagnetic spectrum that derives its characteristics from the material of which the emitting source is made and from the way in which the material is excited. Gaseous spectrum, the spectrum of an incandesoent gas or vapor, under moderate, or especially under very low, pressure. It is characterized by bright bands or lines. Normal spectrum, a representation of a spectrum arranged upon conventional plan adopted as standard, especially a spectrum in which the colors are spaced proportionally to their wave lengths, as when formed by a diffraction grating. Ocular spectrum. See Spectrum Prismatic spectrum, a spectrum produced by means of a prism. Solar spectrum, the spectrum of solar light, especially as thrown upon a screen in a darkened room. It is characterized by numerous dark lines called Fraunhofer lines. Spectrum analysis, chemical analysis effected by comparison of the different relative positions and qualities of the fixed lines of spectra produced by flames in which different substances are burned or evaporated, each substance having its own characteristic system of lines. Thermal spectrum, a spectrum of rays considered solely with reference to their heating effect, especially of those rays which produce no luminous phenomena. Electromagnetic spectrum (EM): the entire range of wavelengths or frequencies of electromagnetic radiation extending from gamma rays to the longest radio waves and including visible light Mass Spectrum: the spectrum of a stream of gaseous ions separated according to their mass and charge. Juniata College Science in Motion Spectroscope An optical instrument for forming and examining spectra (as that of solar light, or those produced by flames, in which different substances are volatilized), so as to determine, from the position of the spectral lines, the composition of the substance. The spectrum of an element may also be produced by exciting the electrons of the element by passing electricity through it in a vacuum. Wavelength The distance between one peak or crest of a wave of light, heat, or other energy and the next corresponding peak or crest. Juniata College Science in Motion “Chemical Detective activity” Identifying the elements through emission spectra Juniata College Science in Motion Objective Students identify substances based on the visible spectra they emit, gaining familiarity with discrete spectra and their relationship to chemical elements. Precautions: Power Sources for gas lamps are a very high voltage. EXTREME CAUTION SHOULD BE USED TO PLUG AND UNPLUG. STUDENTS SHOULD NOT TOUCH THE LAMPS DIRECTLY. ALWAYS TURN OFF POWER SOURCE AND UNPLUG BEFORE CHANGING THE GAS BULB. The lamps should be on for minimum lengths of time to conserve bulb life. Related Subjects: Astronomy Physical Science Chemistry Students should already know: Vocabulary terms above That each element emits a unique wavelength of light Basic use of a spectroscope (see directions on insert of sprectroscopes) Introduction: When you listen to the radio, cook dinner in a microwave, or watch TV you are using electromagnetic waves. Radio waves, television waves, and microwaves are all types of electromagnetic waves. They only differ from each other in wavelength. Wavelength is the distance between one wave crest to the next. Juniata College Science in Motion Wavelength Crest Trough Waves in the electromagnetic spectrum vary in size from very long radio waves the size of buildings, to very short gamma-rays smaller than the size of the nucleus of an atom. http://imagers.gsfc.nasa.gov/ems/waves3.html Electromagnetic waves can not only be described by their wavelength, but also by their energy and frequency? All three of these things related to each other mathematically. This means that it is correct to talk about the energy of an X-ray or the wavelength of a microwave or the frequency of a radio wave. The electromagnetic spectrum includes, from longest wavelength to shortest: radio waves, microwaves, infrared, optical, ultraviolet, X-rays, and gamma-rays. Visible light/ optical waves are the only electromagnetic waves we can see. We see these waves as the colors of the rainbow. Each color has a different wavelength. Red has the longest wavelength and violet has the shortest wavelength. When all the waves are seen together, they make white light. In a rainbow or the separation of colors by a prism we see the continuous range of spectral colors, the visible spectrum. A spectral color is composed of a single wavelength and can be correlated with the wavelength as shown on a EM chart. Purpose: The purpose of this laboratory assignment is to explore the visual electromagnetic spectrum using a spectroscopes and gas tubes. Juniata College Science in Motion An electromagnetic spectrum is an arrangement of electromagnetic waves according to wavelength and frequency. To realize that each element/gas emits a unique spectrum. Identify these elements/gases by analyzing the spectrum that they emit. Realize that stars are composed of various elements, usually the lighter elements, and these can also be identified by their spectrum. Materials Overhead projector, holographic diffraction grating, and two pieces of 8” × 10” dark paper; Hand-held diffraction gratings or hand-held spectroscopes; Various light sources (light sources with a long thin tube or filament are easier to view): • incandescent bulb (creates a continuous spectrum); • fluorescent tube (coated tubes yield a seemingly continuous spectrum); • “black light” tube (uncoated tube for creating a discrete spectrum); • gas spectrum tubes for different elements, include neon if possible (gas spectrum tubes create discrete spectra); and • gas spectrum tube power supply; Activity Sheet A: “Visible spectra for chemical elements” (Figure 1); Activity Sheet B: “Chemical Detective” (Figure 2); Colored pencils, markers, or crayons. Safety Caution students not to touch the light sources as they may be hot and the gas tubes use high voltages. Students should not insert the gas spectrum tubes in the power supply or change the gas spectrum tubes. Students should not stare directly at the light sources for extended periods of time. Procedure 1. In a darkened room, use the overhead projector, the holographic diffraction grating, and the two pieces of dark paper to project a continuous color spectrum on a wall or screen following the detailed instructions given in “The Visible Spectrum” activity on the back of The Electromagnetic Spectrum poster. 2. Ask students to describe what they see and make a colored drawing in the space provided. Students may compare the spectrum with a rainbow or with light seen through a prism or crystal. If desired, explain that the diffraction grating separates the light according to wavelength. Juniata College Science in Motion The activity 1. Hold the hand-held diffraction gratings or spectroscopes to your eye. In a darkened room, view the incandescent source through their gratings (or spectroscopes). 2. Record observations. 3. View the sources that produce a discrete spectrum (the black light and one of the gas tubes, saving the neon tube for step 2 below). 4. Light source will be turned on around the room at various locations for student viewing. DO NOT TOUCH THEM OR ATTEMPT TO TURN THEM OFF!. 5. Draw the spectra you see and explain how the spectra produced by the black light and the gas tube differ from the one produced by the incandescent light. 6. Describe any relationship that might exist between the colors viewed in the spectrum and the appearance of the light source to our eyes (white light has all colors; black light has purple, blue, and green but not much orange or red; a hydrogen tube looks purple and has purple, blue, and red). 7. Consider electromagnetic fingerprints and discuss why they are important in identifying individual elements. Spectra are analogous to fingerprints: Each chemical element and molecule produces a unique pattern of spectral lines. This pattern of lines can be used to identify the presence of a particular element or molecule in an unknown substance. 8. Identity, illuminate the following gas tubes positioned around the room and justify your predictions. Juniata College Science in Motion 9. Use the following chart to help you identify the spectra for the various elements and have match the lines they see from the neon gas tube to the chart. 10. Describe how you would use the previous investigation to determine what elements might be burning from the flame colors or what elements a star might be made of. Juniata College Science in Motion Assessment Arrange students into new groups. Give each group a copy of Figure 2. Have each group work as a team to solve the mystery and submit a written report discussing their solution, the evidence they gathered that led them to the solution, and how they used spectroscopic techniques to solve the crime. Consider stressing that more than one “criminal element” was involved. Note that the “aliases” of the criminal elements are their chemical symbols, and the “perpetrators” are argon and sodium. Reinforcing concepts The light that we see with our eyes represents only a small portion of the electromagnetic spectrum. Developing the technology to detect and study other portions of the electromagnetic spectrum has had a tremendous impact on astronomy, where scientists must use the properties of light to learn about objects that are too far away to visit. NASA educational materials use astronomical data and the excitement of space exploration to reinforce fundamental science concepts such as the electromagnetic spectrum and motivate interest in science and technology. Juniata College Science in Motion Assignment: Use colored pencils or crayons to complete the following assignment. 1. Draw the colors of the spectra you see in Incandescent Light 2. Explain how the spectra produced by the black light and the gas tube differ from the one produced by the incandescent light. 3. Draw the colors of the spectra you see in the gas tube. 4. What is your hypothesis as to the elemental gas found in the tube? 5. Draw the colors of the spectra you see in the gas tube. Juniata College Science in Motion 6. What is your hypothesis as to the elemental gas found in the tube? 7. Draw the colors of the spectra you see in the gas tube. 8. What is your hypothesis as to the elemental gas found in the tube? 9. Draw the colors of the spectra you see in the gas tube. 10. What is your hypothesis as to the elemental gas found in the tube? Juniata College Science in Motion 11. Draw the colors of the spectra you see in the gas tube. 12. What is your hypothesis as to the elemental gas found in the tube? 13. Draw the colors of the spectra you see in the gas tube. 14. What is your hypothesis as to the elemental gas found in the tube? 15. Draw the colors of the spectra you see in the gas tube. Juniata College Science in Motion 16. What is your hypothesis as to the elemental gas found in the tube? 17. Draw the colors of the spectra you see in the gas tube. 18. What is your hypothesis as to the elemental gas found in the tube? 19. Draw the colors of the spectra you see in the gas tube. Juniata College Science in Motion 20. What is your hypothesis as to the elemental gas found in the tube? 21. Which of the previous element/gases would you most likely find in our Sun, a medium sized and aged star? 22. Draw the probable spectrum lines for our Sun. 23. What might the spectrum lines look like for a very old star, such as a red giant? 24. What would be the main element/gas of the red giant? Juniata College Science in Motion Teacher’s Page PA Assessment Standards: Physical Science, Chemistry and Physics 3.4.7A Describe concepts about the structure and properties of matter. 3.4.7B Relaate energy sources and transfers to heat and temperature. 3.4.7D Describe essential ideas about the composition and structure of the u niverse and earth’s place in it. 3.4.10D Explain essential ideas about the composition and structure of the universe. Technological Devices 3.7.7 Use appropriate instruments and apparatus to study materials. 3.7.10 Apply appropriate instruments and apparatus to examine a variety of objects and processes. Preparation: Show students the incandescent light source, the black light source, and one of the gas tubes other than neon. (To work with gas spectrum tubes, follow the manufacturer’s directions. Gently insert the tube into the power supply. Then, briefly turn the power supply on to illuminate the gas. Turn the power supply off immediately after student viewing to prolong the life of the tube.) Briefly turn on each source so students can see the color of the light, while instructing students not to stare directly at the light sources for long periods of time. Turn each source off after it has been viewed. Ask students to list three to five questions they have about what they see. Discuss that they will use the diffraction gratings (or spectroscopes) to view the light from each source and ask them to predict whether each light source’s spectrum will be similar to or different from that of the overhead projector Students should reconfirm that white light can be diffracted into a continuous color spectrum as was demonstrated at the beginning of the activity. Follow directions provided with the spectroscopes and gas tubes and power source! Juniata College Science in Motion The electromagnetic spectrum Light, or electromagnetic radiation, comes in many forms. There are radio waves, microwaves, infrared light, visible light, ultraviolet light, X-rays and gamma rays, all of which form what is known as the 'electromagnetic spectrum' The electromagnetic spectrum is subdivided into seven regions according to wavelength. Each portion of the spectrum interacts with matter in a slightly different way and is given a different name. From longest to shortest wavelength the seven divisions are: Radio (wavelengths greater than 0.3 metres) Earth's atmosphere hides most electromagnetic radiation from space except visible light, certain infrared frequencies and radio waves. For this reason, we can place radio telescopes on Earth's surface and radio astronomy was the first non-optical study of radiation from space. A number of the most massive galaxies were found to be extremely powerful sources of radio waves. Radio astronomy led to the discovery of pulsars which pulse regular radio emissions. Microwaves (wavelengths between 1 millimetre and 0.3 metres) Earth's atmosphere begins to shield radiation from us. The most important form of microwave radiation in astronomy is called the Cosmic Microwave Background (CMB). Discovered in 1965, CMB comes from all parts of the Universe with the same intensity. CMB became solid evidence for the 'Big Bang' theory, which predicted that the shockwave of the primeval explosion would be still detectable. ESA's Planck mission will study the CMB and thus will be seeing the Universe as it was almost at its beginning. Infrared (wavelengths between 700 nanometres – 1 millimetre) The primary source of infrared radiation is heat. The higher the temperature, the faster the atoms and molecules in an object move and the more infrared radiation. The first infrared space mission was IRAS (Infrared Astronomical Satellite) which detected about 350 000 infrared sources. Later, ESA's Infrared Space Observatory (ISO) made important studies of the dusty regions of the Universe. ESA's Herschel mission will build on this work. Visible (wavelengths between 400 – 700 nanometres) Until 1945, most astronomy was optical. This meant studying a very small range of wavelengths. It is from these optical wavelengths that most people derive their picture of the Universe, dominated by bright stars and galaxies. Visible light is predominantly released by objects between 2000 and 10 000°C. The NASA/ESA Hubble Space Telescope has a powerful optical telescope on board which enables it to take stunning photographs in real colour. Ultraviolet (wavelengths between 10 – 400 nanometres) As soon as observations from above the atmosphere became possible, the classical techniques of optical astronomy were extended into the ultraviolet. The Sun and other hot objects are sources of ultraviolet radiation. In 1978, the International Ultraviolet Explorer (IUE) was launched. IUE dominated ultraviolet space astronomy for nearly two decades. It generated spectra showing intensities at different wavelengths from selected objects in the sky. Temperatures, motions, magnetism and chemical composition are all discernible in the ultraviolet spectra. X-rays (wavelengths between 0.01 – 10 nanometres) Most of the observable matter in the Universe today is in a hot state, radiating short-wavelength radiation and X-rays. Massive clouds of gas at a very high temperature fill the spaces between galaxies. Whenever a new star is formed, a collapsing cloud of gas reaches temperatures sufficient Juniata College Science in Motion for nuclear reactions to start, powering the star. Conditions in the primeval Universe were very different - with only a few pre-existing molecules and no dust available for cooling, only the most massive clouds could collapse. They would make not stars, but black holes. Theorists suspect that giant black holes may have been among the earliest objects created in the Universe and would have produced X-rays. Two ESA missions, XMM-Newton and XEUS are designed to observe these X-rays. Gamma rays (wavelengths less than 0.01 nanometres) Gamma rays from space are blocked by the Earth’s atmosphere – fortunately for us, because this powerful radiation is lethal. Gamma-ray telescopes in space give evidence for the processes that made the Universe habitable. When a massive star has used up its hydrogen fuel, it ends in a supernova explosion, emitting gamma rays. During this explosion, radioactive elements are formed and ejected into space, decaying or combining to form the other elements. ESA's COS-B satellite (1975-1982) created a catalogue of gamma-ray sources. ESA's Integral spacecraft, launched in 2002, takes this work forward, studying the phenomenon known as 'gamma-ray bursts'. http://www.esa.int/esaSC/SEM0W1T1VED_index_0.html Observations: Seeing in visible wavelengths Even a casual glance into a clear night atmosphere reveals that at visible wavelengths, stars dominate our surrounding sky. Visible light is the predominant electromagnetic radiation released by objects with temperatures of between 2000 and 10 000°C. Orion Nebula's Trapezium cluster Stars come in a variety of masses, with the vast majority containing between one tenth and ten times the mass of our Sun. The different masses determine how efficiently they generate energy and this gives rise to the surface temperature. Lower temperature stars shine with red light and high-temperature stars are blue or white. Being yellow, our Sun is a middle temperature star measuring around 6000°C. Juniata College Science in Motion All the stars visible to the naked eye are part of our galaxy, the Milky Way. However, thousands of millions of other galaxies stretch throughout space and these are mostly studied using visible wavelengths. The visible wavelengths are also the realm of the emission nebulae. They are glowing clouds of gas and often form some of the most breathtaking objects in the Universe, most often appearing red. This color comes from the predominant emission from nebulae, which is hydrogen gas. Because of the nature of the structure of the hydrogen atom, when it releases energy it does so efficiently at a specific wavelength of red light (656 nanometres). NGC 2264, Cone Nebula These are mostly clouds of gas that surround young, massive stars but some emission nebulae are the death shrouds of old stars. These are historically, but confusingly, called 'planetary nebulae'. Exploding stars also create emission nebulae known as ‘supernova remnants’. Eye-catching celestial helix The most common way for astronomers to analyse visible light from a celestial object is to split it into its constituent wavelengths to form a spectrum. Studying this allows astronomers to analyse the composition and physical condition of the celestial object under consideration. If they are close to a star, dust clouds can reflect visible light. Such clouds usually create blue patches in space, similar to the colour of our daytime sky. http://www.esa.int/esaSC/SEMNFEX5WRD_index_0.html Juniata College Science in Motion The following activities are from FUSE, a joint project of the National Aeronautics and Space Administration and the Johns Hopkins University in collaboration with: Centre National d'Etudes Spatiales (France), the Canadian Space Agency, the University of Colorado, and the University of California, Berkeley. http://fuse.pha.jhu.edu/overview/mission_ov.html They have been reprinted with permission. Exploring Our Universe: From the Classroom to Outer Space I. Spectroscopy Activity #6 SPECTROSCOPY: CHEMICAL DETECTIVE NOTES TO THE TEACHER Grades 8 and up Level: Objectives: Students will explain the process of dispersion, and will identify substances based on the visible spectra they emit. Juniata College Science in Motion Materials: Triangular prisms Hand-held diffraction gratings Various light sources: (Note: if the light source is a long thin tube or filament, you can avoid the need to put a slit in front of the source.) o o Incandescent bulbs (continuous spectrum) fluorescent tubes (coated tubes yield a seemingly continuous spectrum) o "black light" tube (uncoated tube for discrete spectrum) o spectrum tubes for different elements (discrete spectra) Visible spectra for various chemical elements (see attached handout) "Chemical Detective" activity (see attached handout) Procedures: [NOTE: students should have previous exposure to the electromagnetic spectrum and to the concepts of wave refraction and interference.] 1. Use a prism or diffraction grating with an incandescent bulb to project a continuous color spectrum on a wall or overhead screen. Explain that the light is being dispersed, or separated according to wavelength. Have students identify the colors present in the spectrum; explain that there is no set number of colors in the spectrum, but that it is a continuous range of colors. 2. Give students hand held diffraction gratings. Have them view an incandescent source to reconfirm that white light can be separated into a continuous color spectrum. Next have them view a source which produces a discrete spectrum (gas tube, "black light") and have them explain how the spectrum they see is different. Ask them to describe any relationship that might exist between the colors viewed in the spectrum and how the light source looks to our eyes (white light has all colors, "black light" has purple, blue and green but not much orange or red). 3. Ask students to consider fingerprints and explain why they are important. Tell them that spectra can be used just like fingerprints: each chemical element and compound produces a unique pattern of spectral lines. This pattern of lines can be used to identify the presence of a particular element or compound in an unknown substance. Without telling them its identity, illuminate a neon gas tube. Ask students to guess what is in the tube; have them justify their guesses. Have students view the neon gas tube through their diffraction gratings and record the number of spectral lines they view and the color of each line. See whether students notice a relationship between the colors of the spectral lines and the color of the light our eye sees (most of neon's emission lines in the visible range are red and orange, so neon appears red). Give students a copy of the handout "Elemental Spectra" showing visible spectra for Juniata College Science in Motion various elements and have them match the lines they see from the gas tube to the c 4. Arrange students into groups. Give each group a copy of the activity "Chemical Detective." Have each group work as a team to solve the mystery and submit a written report discussing their solution, the evidence they gathered that led them to the solution, and how they used spectroscopic techniques to solve the crime. Discussion: All secondary school physical science texts discuss the phenomenon of light dispersion. High school physics texts usually include a mathematical description of the process by which different wavelengths of light can be separated. Yet the texts seldom offer students activities that reinforce these concepts. This is unfortunate, since a demonstration of spectroscopy techniques almost always produces an "oh, wow!' response. The viewing of a spectrum- a rainbow of colors- is always a memorable experience; it is also a vivid example of light's wave-like properties. 1. Give students diffraction gratings to take home. Have them observe Extensions: light sources in their neighborhood (street lights, business signs, etc.) For each light source they observe, have them record whether the spectrum they viewed was continuous or discrete. For discrete spectra, have students record the number of lines viewed, and the colors of the lines. Have students use the sample spectra from various chemical elements to identify the composition of the light source. (NOTE: there are charts listing the visible spectra for typical light sources such neon, metal halide, sodium vapor and mercurysee Sources. 2. Discuss how a diffraction grating works. Develop the equation relating the wavelength of colors in a spectrum to the dispersion angle and the spacing of the lines on the diffraction grating [ = d sin ]. If available, use spectrographs to measure wavelengths of simple spectral lines. 3. Have students who have learned triangle trigonometry do Activity #4 in Kit II: Tracing Light Through - Understanding Diffraction. This activity teaches the principles on which the FUSE spectrometer is based and tests understanding by asking students to trace light rays from a star through the spectrometer. 4. Have students research the process by which rainbows are formed, and explain the conditions necessary for viewing them. 5. Have students contact fluorescent light manufacturers (check websites like www.sylvania.com or www.ge.com) to find out how fluorescence works and what techniques they use to make fluorescent lights produce a spectrum similar to natural light (sunlight). Also, they can investigate what type of lighting is most appropriate for different situations, and how this relates to the spectral emissions. Juniata College Science in Motion Sources: Flinn Scientific, 1-800-452-1261 (for hand held diffraction gratings) Electro-Technic Products, Inc., 773-561-2349 (for gas spectrum tubes) Arbor Scientific, 1-800-367-6695 ("Night Spectra Quest" chart for identifying typical light sources) CHEMICAL DETECTIVE STUDENT ACTIVITY You are a private eye who stays in business mainly by recovering lost dogs and the occasional runaway pet turtle. You have just learned that last night some devious criminal elements pulled a huge bank robbery. This could be your big break. Crack this case and you'll be famous! You grab your diffraction grating and head to the scene of the crime. What is that strange glowing gas you see in the bank vault? Hold your breath, it's a clue. You look at the gas through the grating. The emission lines you see are like fingerprints. Now you can identify the criminal elements. This is the "perpetrator spectrum": Look at the visible light emission lines of the suspects, shown on the following page. Can you match the lines in the "perpetrator spectrum" to the elements whodunnit? The president of the bank is offering a huge reward to whoever solves this case but is demanding hard evidence against the wrongdoers. Write a report to the president of the bank listing the names and aliases of the perpetrators and, most importantly, explaining in detail how you used techniques of spectroscopy to crack the case. Juniata College Science in Motion Juniata College Science in Motion Cosmic Barcodes Text by Ken Sembach and Bill Blair Graphics by Ken Sembach Astronomers learn about the Universe by observing light from distant astronomical objects, like stars or galaxies. Light contains information, and since it is much easier to observe a star than it is to travel to one, there is clearly a benefit to being able to understand what the light is telling us! One of the main tools for studying light is a device called a spectrograph, which breaks light into its component colors, much like raindrops refract sunlight to produce beautiful rainbows in the sky. When attached to a telescope, a spectrograph becomes a powerful tool for learning about the Universe. Luckily for astronomers, this technique can be used with all kinds of light, not just the visible light that our eyes are sensitive to. Different kinds of light, like infrared, ultraviolet, X-ray light, etc., contain different kinds of information. The FUSE satellite uses light in the far-ultraviolet spectral region, light having wavelengths between 90 and 120 nanometers (1 nanometer = 1 billionth of a meter!). For comparison, visible light ranges from about 400 to 700 nanometers (4000 to 7000 Angstroms). In the absence of any intervening material, the light from a star reaches us unobscured. When the light is dispersed into colors by a spectrograph, it may look something like this continuous spectrum, which has a smooth, gradual change of color, and no breaks or dropouts in the intensity of the light: However, if there are one or more gas clouds between us and the star, this interstellar medium absorbs some of the light before it reaches us. Depending upon what types of Juniata College Science in Motion atoms or molecules are present in the absorbing gas clouds, a number of dark features, or absorption lines, are superimposed on the continuous spectrum emitted by the star, something like this: These absorption lines contain information about the composition of the clouds (the kinds and relative amounts of atoms and molecules in the gas). They also tell us such things as how much gas is in the clouds, the gas density, the temperature of the gas, how fast it is moving toward or away from us, whether there are cold regions embedded in warmer material, and whether there are interstellar dust grains mixed in with the gas. All this from analyzing the light! These absorption features can be thought of as cosmic barcodes, with each type of atom or molecule producing a different barcode signature. One can then think of a spectrograph as a "barcode reader". Once the barcode produced by a gas cloud has been read, astronomers can interpret what the barcode means. Here are examples of the types of barcodes that are produced by the FUSE spectrographs: Juniata College Science in Motion Now it's YOUR turn! See if you can guess which elements have left their mark on this spectrum: Juniata College Science in Motion The Redshift Explained The above diagram shows the spectrum (component colors) of a galactic star. The different wavelengths(the distance from one wavecrest to the next) of light are what human eyes see as colors. The shortest wavelengths appear at the blue end of the spectrum. The longest wavelengths appear at the red end of the spectrum. The numbers on the top measure wavelength in nanometers. The deep black lines in the color spectrum are absorption lines. Each chemical element in a star's atmosphere absorbs a certain color. By looking at the absorption lines in a spectrum, we are able to determine what elements are present in a stars atmosphere (the missing colors are the colors that the elements in the stars atmosphere absorb). Juniata College Science in Motion Notice how the pattern of absorption lines shifts from the blue end to the red end as the galatic star becomes fainter. This is known as the Red Shift. The Doppler Effect is used to explain the Red Shift. The Doppler Effect states that if a source that is emitting waves moves away from us, the wavelength of the waves we recieve from it will be longer. This implies that stars moving away from us will be red shifted(light with longer wavelength appears at the red end of the spectrum). In the 1920's, Edwin Hubble discovered that many galaxies appeared red shifted. This means that these galaxies are moving away from us! The universe is expanding! The idea that the universe is expanding supports the big bang theory. Here are a few explainations of the Red Shift which do not support the big bang. *The following comes from CREATION-EVOLUTION ENCYCLOPEDIA* [1] Gravitational redshifts. Light rays from the stars must travel vast distances to reach us. It has been proven that the pull of gravity, from the stars the light rays pass, could indeed cause a loss in light-wave energy—thus moving that light toward the red on the spectrum. Einstein was the first to predict that gravity would affect starlight, and this was shown to be true in the 1960s.—p. 35. Albert Einstein was the first to predict that gravity would be able to affect the transmission of light. This fact could easily explain the redshifts which have been found.—p. 42. [2] Second-order Doppler shift. It is known that a light source moving at right angles to an observer will be redshifted. Compare this fact with the known fact that all stars are definitely circling galaxies. In addition, many scientists suspect that, just as all planets and stars are kept in position by orbiting, so, for purposes of stability, the entire universe is probably circling a common center!—pp. 35-36. [3] Energy-loss shift. Light waves could themselves lose energy as they travel across the long distances of space. This is called "tired light." The energy-loss shift is probably the primary cause of the redshift.—p. 36. http://www.angelfire.com/nt/fairytales/redshift.html BLUE SHIFT The blue shift is a decrease in the wavelength of the light that is emitted from an object that is moving toward us. This decrease in wavelength makes the object appear to be bluer than it actually is. For example, when a star is travelling towards Earth, its light appears bluer (the light waves are shortened, shortening the wavelength). Compare with red shift. http://www.allaboutspace.com/subjects/astronomy/glossary/indexb.shtml Juniata College Science in Motion Activity: Determining Red-Shift in a Receding Star Instructional Objectives Time Needed for Activity Target Grade Level Materials Background Information & Questions Web Resources Instructional Objectives: Students will 1. 2. 3. 4. manipulate multivariable algebraic formulas, understand velocity, wavelength and frequency, study the Doppler effect, determine the amount of red-shift in light from a receding star. Time Needed For Activity: 45 minutes Target Grade Level: Advanced high school students. Materials: Calculator Dictionary Background Information and Questions: <P< expanding an of consequence a as Hubble by observed red-shift the explain to student physics basic allow techniques spectroscopy stellar with coupled effect Doppler and nature wave understanding Application light. galaxies distant measurements accurate Juniata College Science in Motion careful his from stems discovery Hubble?s century. finding scientific significant most many is was universe that> Part 1: LIGHT WAVES Light is best described as a wave. All waves are characterized by a wavelength and a frequency. The wavelength describes the distance between the crest of each cycle. The frequency is the number of crests that pass any given point every second. Visible light waves range in wavelength from approximately 400 nanometers (400 nm = 400 x 10-9m) for violet colors to about 700 nanometers for red. Correspondingly, violet has a frequency of about 7.5 x 1014 Hz (where 1 Hz is equal to 1 cycle/second) and red has a frequency of 4.3 x 1014 Hz. Light is a type of electromagnetic radiation. Other types of electromagnetic radiation like ultraviolet light, infrared light, radio waves and X-rays also travel in the form of a wave but at wavelengths to which our eyes are insensitive. Sound is not electromagnetic radiation, but sound is a wave as well. Higher pitches are caused by higher frequencies of vibrating molecules that reach your eardrum. Lower pitches are likewise caused by lower frequencies. Questions: Juniata College Science in Motion 1. What color has the longest wavelengths? 2. What color has the shortest wavelengths? 3. What color has more crests of its wave passing a given point in one second? Explain. 4. Look up the prefixes "ultra" and "infra" in the dictionary. Explain why the wavelengths just out of the visible spectrum are referred to as ultraviolet and infrared. Part 2: DOPPLER EFFECT If a wave source is moving, the crests of its waves get bunched together in front of the wave source. If the wave crests are bunched together, their frequency increases. In the case of a sound wave, the pitch is higher. Behind the wave source, the waves spread out and the pitch is lower. In the case of light we use the terms "blue-shift" and "red-shift" to describe how the DOPPLER EFFECT changes the wavelength of the light. Being blue-shifted or redshifted doesn't mean that the light necessarily becomes blue or red. It means simply that the light's wavelength either is shortened (blue shifted) because the object giving off the light is approaching, or is lengthened (red-shifted) because the object is moving away from the observer. Part 3: DETERMINING THE COMPOSITION OF STARS When energized atoms and molecules vibrate, they give off massless light particles called photons. These photons travel as a wave, but because of quantum energy effects, a particular type of atom or molecule gives off only certain wavelengths of photon light. For example, when hydrogen atoms are giving off energy in the form of light, they emit light specifically at wavelengths of 410.2 nm, 434.0 nm, 486.1 nm, and 656.3 nm. This is called the emission spectra of hydrogen. Scientists can use the emission spectra of atoms and molecules to study the composition of stars. Scientists need simply to look very carefully at the intensity and wavelengths of the light given off by the star. A star containing hydrogen, for example, would have intense peaks of energy at 410.17 nm, 434.05 nm, 486.13 nm, and 656.28 nm. These Juniata College Science in Motion hydrogen emission peaks would be in addition to the ones associated with the other elements contained in the star. Part 4: RECEDING STARS In the 1920's, Edwin Hubble, while studying the stars of distant galaxies, found that for some, their emission spectra had peaks at 411.54 nm, 435.50 nm, 487.75 nm, and 658.47 nm. Hubble knew that these wavelengths did not correspond to any known element and that it was not likely that a combination of other elements or molecules was responsible. He did notice that these spectral lines corresponded to hydrogen's emission spectra except that they were all 0.0033 percent longer in wavelength than they should have been for a hydrogen spectra. Hubble deduced that this red-shift must be because of a Doppler effect. His calculations showed that these galaxies must be moving away from earth at 1 x 106 m/s (one million meters per second)! Part 5: VERIFY HUBBLE'S WORK THAT SHOWS THE UNIVERSE IS EXPANDING For all waves, the product of wavelength and frequency gives the velocity of the wave where (lambda) is the wavelength and f is the frequency. v = (lambda) f In the case of light and other electromagnetic radiation, however, the velocity is always fixed at 3 x 108 m/s. This speed of light is assigned the variable c. vlight = c = 3 x 108 m/s, or more precisely, c = 2.99792458 x 108 m/s Use c = 2.9979 x 108 m/s for any calculations below. Also, use the appropriate number of significant digits. Questions: 1. What would the frequency be of a violet light wave with wavelength of 410.17 nm? 2. What frequency is associated with 434.05 nm? 3. What frequency is associated with 486.13 nm? 4. What frequency is associated with 656.28 nm? 5. Imagine the hydrogen atoms in one of Hubble's distant stars emitting a photon of light at a wavelength of 410.17 nm. If that particular photon were headed directly TOWARD Earth, how much CLOSER to us would that photon be after one second? (Recall that the distance, d, that an object travels in a time, t is given by d = vt where v is the object's velocity.) 6. Now if the star that emitted the photon were traveling AWAY from Earth at 1 x 106 m/s, how much FARTHER from Earth would the star be after one second? 7. After one second, what is the distance between our initial photon and its star? Juniata College Science in Motion 8. Our initial photon would be followed by many, many more just like it, each behaving similarly to the first. After one second, how many wave cycles of photons would stretch between the distant star and our initial photon? 9. What is the average wavelength of this photon light? (Hint: Use your answers from 4 and 5 above.) Compare this with what Hubble saw. 10. Convince yourself of the validity of this process by repeating questions 2 through 6 for one more of the hydrogen emission spectra peaks at wavelengths 434.05 nm, 486.13 nm, or 656.28 nm. (Choose (a) the distance the photon travels toward Earth, (b) the distance the star moves away from Earth, (c) the distance between the photon and the star, (d) the number of cycles between the star and the photon, (e) the effective red-shifted wavelength.) 11. If you were driving in a VERY fast car, how would the things you approach look different from normal? How would the things you drive away from look different? Explain. 12. Why do you think Hubble's discovery that the universe is expanding would have been impossible without instruments that could precisely measure emission spectra? http://www.pbs.org/deepspace/classroom/activity2.html Juniata College Science in Motion