POLB_21930_sm_SuppMaterials
advertisement

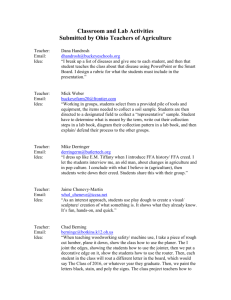
Supporting information 0.42 0.40 0.38 h (1/dh) 0.36 0.34 0.32 eq.1 eq.2 eq.3 0.30 0.28 0.26 0.5 0.6 0.7 0.8 0.9 1.0 cA Figure 1 Comparison of the dependence of exponent λh calculated by Rh~Nλ on cA under different equations of Rh. Rh N N 2 N i 0 j 0( i j ) N N N Rh N ( N 1) Rh N 2 1 rij i 1 j 1( i j ) N i 1 j 1( i j ) 1 rij eq.1 1 rij eq.2 eq.3 According to reference 1, the equation 1 and 3 are given in the page of 243 and 21 respectively. However, both eq.1 and eq.3 should be more suitable for infinitely large polymers. For small molecules, there may be large deviations because the calculated number in numerator and denominator is not identical. In order to keep the consistency of numerator and denominator, we proposed the eq.2 in this work. Since the degree of polymerization is finite for all hyperbranched molecules obtained in our simulation, we think eq.2 is more accurate. With the growing up of the polymers, the discrepancy caused by various equations gradually decreases. This trend can be seen in Figure 1, with the increase of cA, the λhs calculated by different equations gradually close to each other. For infinitely large polymers, the results calculated by these three equations should be the same. 4 c0=0.1 4 c0=0.3 3 Rhz* c0=0.5 3 c0=0.7 2 Rgz* c0=0.9 1 0.2 2 0.4 0.6 0.8 1.0 cA 1 0.2 0.4 0.6 0.8 1.0 cA Figure 2 Dependence of the dimensionless z-average radius of gyration, Rgz*, and z-average hydrodynamic radius Rhz*, on the conversion of A groups, cA, under various monomer concentrations, c0, for AB4 type polymerization. 5 4 k12=1/10 k12=1/3 3 Rhz* 4 k12=1 k12=3 2 k12=10 Rgz* 3 1 0.2 0.4 0.6 0.8 1.0 cA 2 1 0.2 0.4 0.6 0.8 1.0 cA Figure 3 Dependence of the dimensionless z-average radius of gyration, Rgz*, and z-average hydrodynamic radius Rhz*, on the conversion of A groups, cA, at various reactivity ratios, k12, with monomer concentration c0 = 0.2 for AB2 type polymerization. 1.3 c0=0.1 c0=0.3 1.2 c0=0.5 1.1 c0=0.7 c0=0.9 (Rgz/Rhz) 1.0 0.9 0.8 0.7 0.6 0.5 0.2 0.4 0.6 0.8 1.0 cA Figure 4 Dependence of the ratio of z-average radius of gyration, Rgz, to z-average hydrodynamic radius, Rhz, γ = Rgz/Rhz on conversion of A groups, cA, at various monomer concentrations, c0, with the reactivity ratio k12 =1 for AB2 type polymerization. 1.2 1.1 (Rgz/Rhz) 1.0 0.9 c0=0.1 0.8 c0=0.3 c0=0.5 0.7 c0=0.7 c0=0.9 0.6 0.5 0.2 0.4 0.6 0.8 1.0 cA Figure 5 Dependence of the ratio of z-average radius of gyration, Rgz, to z-average hydrodynamic radius, Rhz, γ = Rgz/Rhz on conversion of A groups, cA, at various monomer concentrations, c0, for AB4 type polymerization. 1.4 k12=1/10 1.3 k12=1/3 1.2 k12=1 k12=3 (Rgz/Rhz) 1.1 k12=10 1.0 0.9 0.8 0.7 0.6 0.5 0.2 0.4 0.6 0.8 1.0 cA (a) 1.3 1.2 1.1 (Rgz/Rhz) 1.0 0.9 k12=1/10 k12=1/3 0.8 k12=1 k12=3 0.7 k12=10 0.6 0.5 0.2 0.4 0.6 0.8 1.0 cA (b) Figure 6 Dependence of the ratio of z-average radius of gyration, Rgz, to z-average hydrodynamic radius Rhz, γ = Rgz/Rhz on the conversion of A groups, cA, at various reactivity ratios, k12, with monomer concentration (a) c0 = 0.2 and (b) c0 = 0.7 for AB2 type polymerization. (a) (b) (c) Figure 7 (a) Schematic illustration of the 3d bond fluctuation lattice model of H. Deutsch and R. Dickman.2 (b) Conformation of an AB2 type monomer. (c) Conformation of an AB4 type monomer. In this model each ABn (n=2, 4) unit takes up eight lattice sites (one cubic cell). In Figure 7 (b) and (c), each bead represents a unit which can be classified as linear (orange), branched (gray) and terminal (blue) units. The difference between AB2 and AB4 type monomers can be seen from the gray unit surrounded by a black cycle. AB2 type monomer can connect with 3 other units while AB4 type monomer can connect with 5 other units. Reference 1. Grosberg, A. Y.; Khokhlov, A. R., Statistical Physics of Macromolecules. AIP Press: New York, 1994. 2. Deutsch, H.; Dickman, R. J Chem Phys 1990, 93, 8983-8990.