supplementary
advertisement
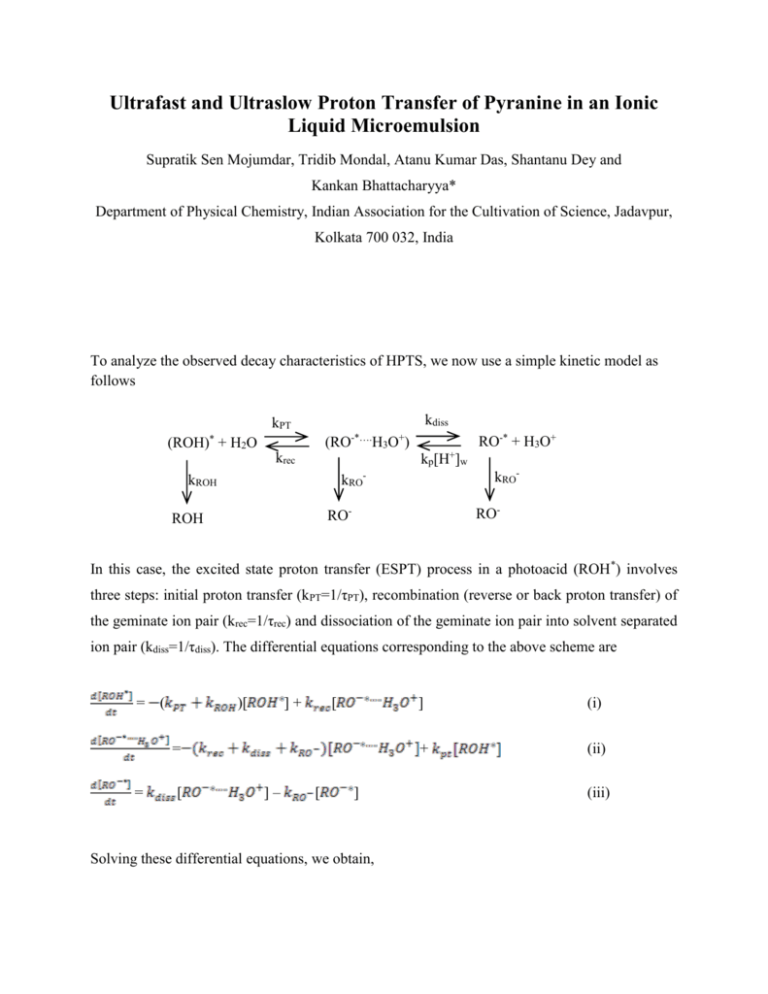
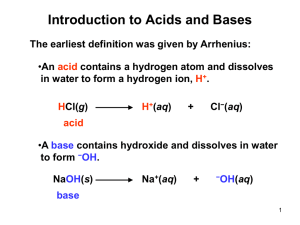
Ultrafast and Ultraslow Proton Transfer of Pyranine in an Ionic Liquid Microemulsion Supratik Sen Mojumdar, Tridib Mondal, Atanu Kumar Das, Shantanu Dey and Kankan Bhattacharyya* Department of Physical Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata 700 032, India To analyze the observed decay characteristics of HPTS, we now use a simple kinetic model as follows kdiss kPT -*…. (ROH)* + H2O (RO RO-* + H3O+ + H3O ) kp[H+]w krec kRO- kRO- kROH RO- RO- ROH + In this case, the excited state proton transfer (ESPT) process in a photoacid (ROH *) involves three steps: initial proton transfer (kPT=1/τPT), recombination (reverse or back proton transfer) of the geminate ion pair (krec=1/τrec) and dissociation of the geminate ion pair into solvent separated ion pair (kdiss=1/τdiss). The differential equations corresponding to the above scheme are = ( )[ ]+ [ = = [ ]– [ ] Solving these differential equations, we obtain, ] (i) + (ii) (iii) = + = + + (iv) (v) where τ1 = τ4 and τ2 = τ5 According to the scheme discussed above, the decay of both the protonated form (430 nm) and deprotonated form (540 nm) should be a sum of three exponentials.