α decay equations
advertisement
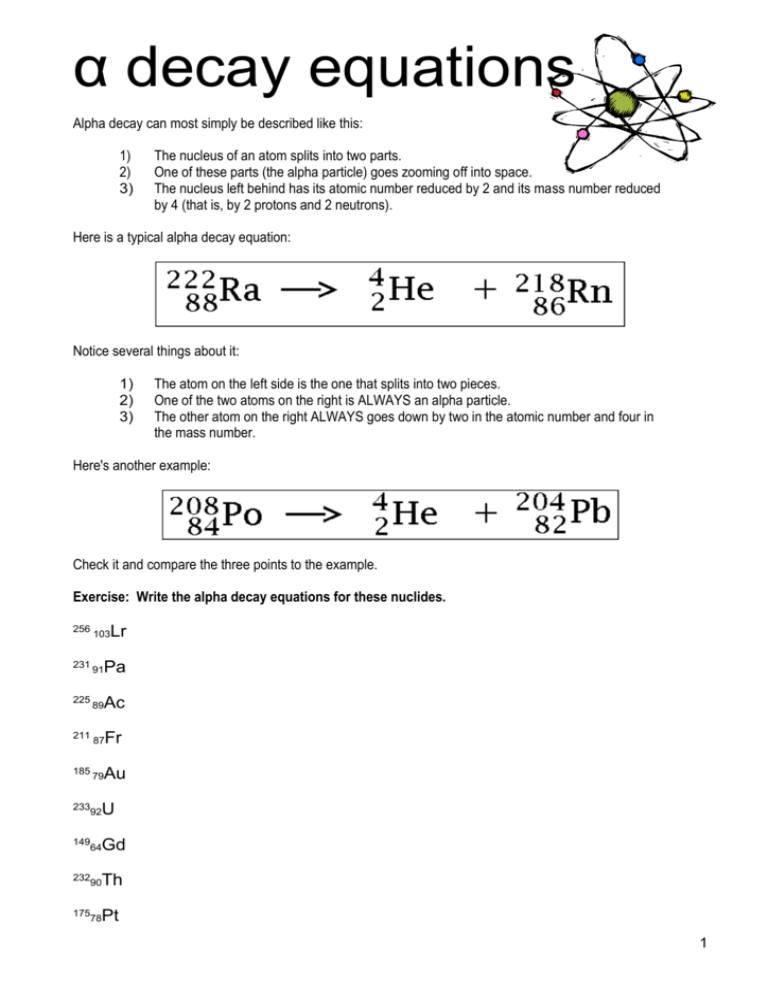
α decay equations Alpha decay can most simply be described like this: 1) 2) 3) The nucleus of an atom splits into two parts. One of these parts (the alpha particle) goes zooming off into space. The nucleus left behind has its atomic number reduced by 2 and its mass number reduced by 4 (that is, by 2 protons and 2 neutrons). Here is a typical alpha decay equation: Notice several things about it: 1) 2) 3) The atom on the left side is the one that splits into two pieces. One of the two atoms on the right is ALWAYS an alpha particle. The other atom on the right ALWAYS goes down by two in the atomic number and four in the mass number. Here's another example: Check it and compare the three points to the example. Exercise: Write the alpha decay equations for these nuclides. 256 231 225 211 185 Lr 103 Pa 91 Ac 89 Fr 87 Au 79 U 233 92 Gd 149 64 Th 232 90 Pt 175 78 1 β decay equations Beta decay is somewhat more complex than alpha decay. Simplified: 1) A neutron inside the nucleus of an atom breaks down, changing into a proton. 2) It emits an electron which goes zooming off into space. 3) The atomic number goes UP by one and mass number remains unchanged. Here is an example of a beta decay equation: Some points to be made about the equation: 1) The nuclide that decays is the one on the left-hand side of the equation. 2) The order of the nuclides on the right-hand side can be in any order. 3) The beta particle is written as an electron with –1 protons. Here is another example of a beta decay equation: Notice that all the atomic numbers on both sides ADD UP TO THE SAME VALUE and the same for the mass numbers. Exercise: Write out the full beta decay equations for these nuclides. 6 He 2 Na 24 11 201 52 42 90 Fe 26 K 19 Sr 38 239 247 82 99 Au 79 Np 93 Am 95 Br 35 Tc 43 2 Write and balance the equations for the following reactions. 1. Neutron initiated fission of U- 235 results in the release of neutrons, the formation of Cs- 144 and Rb-90. 2. Neutron initiated fission of U- 235 results in the release of beta particles, the formation of Sr- 90 and the release of Ce-146. 3. Neutron initiated fission of U- 235 results in the release of x neutrons, one (1) beta particle, and the formation of Br- 87 and Ce-146. 4. Neutron initiated fission of Pu- 239 gives x neutrons, La- 145 and Rb-92. Answers Alpha (α) decay equations Beta decay equations Nuclear fission + 23592U → 144 55Cs + 90 37 Rb + 2(10n) 235 1 90 146 0 92U + 0n → 38Sr + 58 Ce + 4( -1e) 1 235 87 146 1 o 0n + 92U → 35Br + 58Ce + 3( 0n) + -1e 1 239 145 92 1 0n + 94Pu → 57La + 37Rb + 3( 0n) 1 0n 3 The ultimate balancing act! A few people asked for extension exercises — something a little harder. Nucelar Decay Sequence of Uranium Uranium is a common element in the earth's crust and is widely distributed in rocks such as granite and basalts.. Since 238 U is much more abundant than 235 U, we will look only at the decay sequence for 238 U. The half-life for each Notice on the table below I've adopted the E shorthand for ten to a power. Radioactive Decay Sequence of 238 U 238 U alpha 4.41 E 9 year 234 Th beta 24.1 days 234 Pa beta 1.18 min 234 U alpha 2.48 E 5 year 230 Th alpha 8.0 E 4 year 226 Ra alpha 1.62 E 3 year 222Rn alpha 3.82 day 218 Po alpha or beta 3.05 min 214 Pb (from alpha) beta 26.8 min 218 At (from beta) alpha 2 sec 214 Bi beta or alpha 19.7 min 214 Po alpha 1.6 E -4 sec 210 Tl beta 1.32 min 210 Pb beta 19.4 year 210 Bi beta or alpha 5.0 day 210 Po alpha 138 day 206 Tl beta 4.2 min 206 Pb stable See if you can write and balance some of the equations are part of this sequence of decay. If you need more help, read the additional information on the next page! Notice that several isotopes may have two possible decay paths (alpha or beta), but that both routes lead ultimately to the same product, i. e., 206 Pb. Notice that U238 is the parent of 234 U by way of Th and Pa via an alpha and two beta decays. As the decay sequence proceeds, it is not unusual for the atomic number to move backwards and forwards on the periodic table several times. 4 About the decay of uranium-238 Radon gas, like carbon-14 gas, is completely natural. It forms during the decay of uranium-238, an element with a fairly interesting decay sequence. 1. Start with a uranium-238 atom. This atom has 92 protons and 146 neutrons. It has a half-life of 4.5 billion years. When it decays it emits an alpha particle, leaving behind a thorium-234 atom. 2. A thorium-234 atom has 90 protons and 144 neutrons. It has a half-life of 24.5 days. When it decays it emits a beta particle and a gamma ray, leaving behind a protactinium234 atom. 3. A protactinium-234 atom has 91 protons and 143 neutrons. It has a half-life of 269,000 years. When it decays it emits a beta particle and a gamma ray, leaving behind a thorium230 atom. 4. A thorium-230 atom has 90 protons and 140 neutrons. It has a half-life of 83,000 years. When it decays it emits an alpha particle and a gamma ray, leaving behind a radium-226 atom. 5. A radium-226 atom has 88 protons and 138 neutrons. It has a half-life of 1,590 years. When it decays it emits an alpha particle and a gamma ray, leaving behind a radon-222 atom. That radon atom is a gas atom, and it has a half-life of only 3.825 days. Accumulations of radon atoms from the natural nuclear decay of uranium-238 is where radon gas comes from. That means that radon gas concentrations are higher where uranium is plentiful in the soil. For completeness, here is the rest of the sequence: 1. radon-222, with a half-life of 3.825 days, emits an alpha particle to become polonium-218. 2. polonium-218, with a half-life of 3.05 minutes, emits an alpha particle to become lead214. 3. lead-214, with a half-life of 26.8 minutes, emits a beta particle and a gamma ray to become bismuth-214. 4. bismuth-214, with a half-life of 19.7 minutes, emits either an alpha particle or a beta particle and a gamma ray to become either thallium-210 or polonium-214. 5. polonium-214, with a half-life of a 150 microseconds, emits an alpha particle to become thallium-210. 6. thallium-210, with a half-life of 1.32 minutes, emits a beta particle to become lead-210. 7. lead-210, with a half-life of 22 years, emits a beta particle and a gamma ray to become bismuth-210. 8. bismuth-210, with a half-life of five days, emits a beta particle to become polonium-210. 9. polonium-210, with a half-life of 138 days, emits an alpha particle and a gamma ray to become lead-206. 10. lead-206 is a stable isotope of lead. 5 Practice balancing nuclear equations! 1. 2. Complete and balance the following equations: 1 4 He 2 2 1 n 0 a. 7 Li 3 + 1 H b. 3 H 1 + 1 H c. 14 C 6 14 7N d. 9 Be 4 4 + 2 He + 12 C + 6 lead-214 decays by beta emission 3. bismuth-214 decays by beta emission 4. polonium-214 decays by alpha emission The Hint: To do these equations you will need to use a periodic table and work out the name for the element which is produced! ABC of balancing equations: A. Work out your reactants and your products. Write down the parts you know. Use a periodic table if necessary! B. Check mass numbers. Mass is always conserved, so the total mass number on each side must be equal. C. Check atomic numbers. Total on each side must be equal. If you can’t get the numbers to balance then remember, you might need to allow for multiple reactants. For example, three alpha particles: 3(42He) 6 Practice balancing nuclear equations! Complete these nuclear equations by writing the symbols of the missing particles. a) 211 b) 220 82 Pb → o –1e + 86Rn → 4 2He + Write a balanced nuclear equation for each of these changes. (a) Alpha emission from plutonium-242. (b) Beta emission from magnesium-28. (c) Electron capture by argon-37. Write the symbols, including the atomic number and mass number, for the radionuclides that would give each of these products. (a) Fermium-257 by alpha emission. (b) Bismuth-211 by beta emission. Cesium-137, 13555Cs, is one of the radioactive wastes from a nuclear power plant or an atomic bomb explosion. It emits beta and gamma radiation. Write the nuclear equation for the decay of cesium-137. Marie Curie(1867-1934) earned one of her Nobel prizes for isolating the element radium, which soon became widely used to treat cancer. Radium-226, 22688Ra, is an alpha and gamma emitter. Write a balanced nuclear equation for the decay of radium-226. 7 Half-Life When unstable nuclei undergo radioactive decay, their decay rate is not steady. Instead, they have a half-life (t1/2), which is the amount of time required for half of the reactant to disappear. For example, Iodine-131 is unstable and under beta particle emission, it becomes Xenon-131. It has a half life of roughly 8 days. So if we had a 4 gram sample of Iodine-131, in 8 days, there would be 2 grams left. In another 8 days, there would be 1 gram. Iodine-131 Iodine-131 2gms Iodine-131 1gm Iodine-131 Xenon-131 Xenon-131 4gm Xenon-131 Day 1 After 8 days After 16 days After 24 days 3 Half-Life Table Nuclide Half-life Decay Type 6 2 He 0.802 seconds Beta-minus 1.3 minutes Alpha and Gamma 5730 years Beta-minus 7.1 x 10 8 years Alpha and Gamma 227 92 U 14 6 C 235 92 U Test your understanding An archeologist extracts a sample of carbon from an ancient axe handle and finds that it emits an average of 10 beta emissions per minute. She finds that the mass of the carbon from a living tree emits 40 betas per minute. Knowing that the half life of carbon-14 is 5730 years, she concludes that the age of the axe handle is about a) 2865 years c) 11460 years b) 5730 years d) 17190 years 8 COLD WAR HANGOVER PLUTONIUM HEADACHE At the Plutonium Finishing Plant at Hanford, wastes are heated to 1,000 degrees Celsius in this chamber to remove moisture, eliminate explosive gases and reduce volume. When the Cold War ended, America's nuclear-warhead-production system shut down so rapidly that weapons-grade isotopes were left stranded, so to speak, on the assembly line. The United States had produced 100 finished tons of plutonium from which to fashion warheads. Fifty-three of those tons were made at Hanford. Another 26 tons were in intermediate steps before weapons fabrication. Dealing with these leftovers has become one of the top cleanup priorities at Hanford Site in Eastern Washington. The Seattle Times recently got a rare peek at the plutonium-storage vaults there, encased in a windowless concrete building and locked behind 5,000-pound doors. Hanford's Plutonium Finishing Plant is the factory where liquid-nitrate solutions of plutonium from the nuclear reservation's Purex Plant were turned into palm-sized silvery "buttons" that could be machined into warheads. Each button cost $2.8 million to produce, and the laborious process of going from liquid to solid metal took about two weeks. Technicians used soft gold drills to probe button interiors, testing for quality. "This is probably the most secure place in all of Hanford," manager Rick Redekopp said as we laboriously "suited up" to enter the finishing plant. The plutonium was manufactured on a conveyer belt shielded by metal and glass and accessed by "glove boxes," or windows fitted with shielded gloves through which workers could purify the metal. Abrupt shutdown left a residue of plutonium and chemicals along the gritty conveyer line. Now workers are carefully scraping off the gunk, heating it in small ovens to 1,000 degrees Celsius to burn off potentially explosive gases and reduce its volume, and then packing it in lead-lined cans, one inside another inside another. They look like large food cans. If all this waste were stacked together, the concentrated radioactivity could spark a chain reaction and a fire or meltdown. Accordingly, the cans are taken to the plutonium storage vault and stored inside lockers, each can on its own bracket to keep it from coming into contact with the others. They will remain at Hanford for the foreseeable future. While the plutonium will persist for tens of thousands of years, no decision has been made about its long-term fate. It could be turned into glass and buried, or refabricated into reactor fuel. The storage vault is monitored by the International Atomic Energy Agency, the same United Nations body that polices Iraq. "We can't move it without them knowing about it," Redekopp said. Caution about radiation soars as one nears the plutonium vault. One passes several checkpoints, is escorted by an armed guard, and is offered a lead vest plus two electronic radiation dosimeters, another called a "pencil," and three radiation badges. Next to the abandoned reactors which were used to make plutonium from uranium fuel rods, water storage basins hold 300 tons of weapons-grade fuel and 1,800 tons of ordinary reactor fuel. New doors to prevent the 40-year-old pools from breaching in an earthquake have been installed. Meanwhile, plans are being completed to move this mess to dry storage, where it is less likely to contaminate the environment. http://seattletimes.nwsource.com/trinity/articles/closer3.html 9 Acknowledgements http://dbhs.wvusd.k12.ca.us/Radioactivity/Writing-Alpha-Beta.html http://www.chemcool.com/regents/nuclearchemistry/nuclearchemistry.htm#skills%20students%20should %20be%20able%20to%20do http://www.howe.k12.ok.us/~jimaskew/cnucler.htm http://library.thinkquest.org/3659/nucreact/radioactivity.html http://www.howstuffworks.com/radon1.htm Useful links About atomic reations, fission and fusion http://library.thinkquest.org/17940/texts/atom/atom.html?tqskip1=1&tqtime=1102 http://www.sciencenet.org.uk/database/Physics/Atomic/p00435c.html About atomic bombs http://www.atomicarchive.com/sciencemenu.shtml Demo of nuclear fission http://www.atomicarchive.com/Fission/Fission1.shtml Links to sites and practice quizzes http://www.chemtopics.com/unit11/unit11f.htm 10 Name: Revision quiz! Just so that I can see how you are going. Name three types of emissions which frequently result from nuclear reactions. Which two types of emissions are particle emissions? Write the chemical symbols for: (a) an alpha particle (b) a beta particle (c) a neutron (d) a proton. What is nuclear fission? What is nuclear fusion? Complete and balance the following equations: + 4 2He → a) 14 7N 17 b) 24 11Na → 24 12Mg + c) 27 13Al + 10n → 24 8O + 11Na + What sort of reaction is (b)? What sort of reaction is (c)? Strontium-90, one of the most hazardous isotopes produced in nuclear fission, has a half-life of 29 years. Approximately what fraction of a Strontium-90 sample will remain after (a) 58 years and (b) 15 years? 11 Some common fusion reactions of hydrogen isotopes are shown in incomplete forms. Can you complete them? Write balanced nuclear equations for each of the following artificial nuclear transmutations. The brackets indicate the particle that causes the transmutation and the ejected particle. a) Li6 (n, alpha) b) B10 (n, alpha) Complete and balance the following equations: a) b) c) 12 13