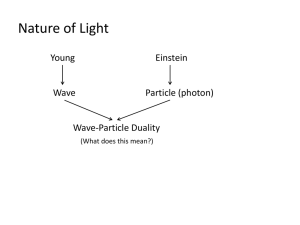
A particle is a discrete unit of matter having the attributes of mass
advertisement

Orbitals, Bonds, Interactions Part 1. Easy Quantum (2.1.07) Introduction I am handing out several readings that you can regard as background, or, if you need no background (ha), as something to skim until you get to the places where I have dropped questions for you to answer that probably cannot be answered definitively by anyone. In all cases, these readings should give you a list of the material I expect you to be familiar with. As I will continue to remind you, students in this class have varied backgrounds, so if this seems like something you just had in an entire semester-long class at a higher level, don’t worry about it (unless you really think you understand anything about it). When I give you handouts on biochemistry and biomacromolecules, the tables will be turned for those who have had biology but little physics. These notes on the rudiments of quantum theory, taken from some web tutorials and my own insertions and deletions, are for students who have not had recent background experience with waves or blackbody radiation or quantum mechanics, and it is only meant to remind you or introduce you to these things at an elementary level. For example, these notes only take you up to the development of the time-independent Schrodinger equation using the idea of the deBroglie wavelength for particles, although most of the material is much more elementary. Some (e.g. the example solutions to Schrodinger’s equation for the particle in the box or related potentials), are deeper than they appear. If you are a student with background in these areas, you may be surprised to read through the material anyway, either as a refresher, or to see how differently you might view these “elementary” topics today. I will try to convert units to cgs (cm instead of m) but probably missed some spots--the rest of the world is mks/SI. I have also left in a few links that you can access from this document if you are interested. We want to work through the topics in Lunine sections 3.2, 3.3, 3.4 by next week. There are some things that will come up so many times that we might as well go over them at different levels now. This writeup, which will be very light reading for some of you and new to others, has headings that tell you what I expect you to become familiar with: Waves and light; blackbody radiation; quantization and matter waves; the hydrogen spectral lines; the Bohr model emerges; orbitals (elementary description--you will be receiving a reading packet that goes over atomic orbitals in gory detail); electron spin; standing wave modes in a plucked string; standing waves in the hydrogen atom; unbound states; physical significance of the wave function; quick derivation of Schrodinger’s equation; orbitals (again); the quantum numbers; the angular momentum quantum number (pictures of orbitals here); magnetic quantum number; electron spin and the exclusion principle. Following this handout will come one that is a little more challenging, that reminds you or informs you in more detail how the orbitals of atoms are labelled to reflect the quantum numbers that are introduced here. The last section ends with a question about what portions of quantum theory really are important in determining the chemical characteristics of macromolecules, and whether the repulsive/attractive nature of the Coulomb force can be derived from first principles, using quantum theory or anything else, or is stuck in because we observed it first. The stars of the show: Here are the main characters in this play; you may notice that the comic and actor Robin Williams is a direct descendant of Erwin Schrodinger, although Williams rarely speaks of it due to his shame for not pursuing a career in physics. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Lord Kelvin Quic kTime™ and a TIFF (Unc ompres sed) dec ompres sor are needed to see this pic ture. Heisenberg QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Maxwell QuickTime™ and a QuickTime™ and a TIFF (Uncomp resse d) de com press or TIFF (Unc ompressed) decompres sor are nee ded to s ee this picture. are needed to see this picture. Planck Einstein deBroglie QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Bohr Dirac Schrodinger QuickTime™ and a TIFF (Uncompress ed) dec ompres sor are needed to s ee this pic ture. Waves and Light A particle is a discrete unit of matter having the attributes of mass, momentum (and thus kinetic energy) and optionally of electric charge. This definition is mostly for fundamental particles; for “macroscopic” or “mesoscopic” (the realm of interest to us, inbetween micro and macro) particles the particle can have other attributes like a measure of its size, shape, composition, etc. But for now we treat everything like a point particle, since it is the electron we are after. We want to understand how molecular orbitals come about--that is our main goal (and the topic of Lunine, sec. 3.2) before learning about chemical bonding (Lunine, sec. 3.3), intermolecular forces (Lunine, almost nothing), biomolecules (Lunine, Chapter 4) and more. But first we have to understand the orbitals of electrons in atoms, and for that we need to be comfortable with certain concepts. A wave is a periodic variation of some quantity as a function of location or time. For example, the wave motion of a vibrating guitar string is defined by the displacement of the string from its center as a function of distance along the string. A sound wave consists of variations in the pressure with location. Waves are often represented as trigonometric functions like a sine wave, so you might write a wave as A0 sin(t) in time or A0 sin(kx) in space, where is the frequency of the wave (how many waves pass a point per second) and k is a spatial frequency called the wavenumber, k=2/; it often takes a while to get used to wavenumber, so think about it now. Waves are so widespread in nature that they form a fundamental part of just about every physics and astronomy course, with more types of waves than you have probably ever heard of. (In fluid dynamics, for example, there are dozens of types of waves; just in planetary meteorology the discussions are dominated by Rossby waves, gravity waves, baroclinic waves,...) The importance of a wave representation has another, practical, twist: Many processes (only those that don’t involve large amplitudes) can be represented in a convenient way as a superposition of many waves of different wavelengths, which is called the Fourier theorem. In this case it is common to see a wave represented as exp(kx) or exp(t); if you do not understand the relation between exponentials and trig functions, i.e. that they can both be used to represent waves, find out about it now. Below we will derive the Schrodinger equation by representing an electron probability wave as an exponential, so get used to it now. For any kind of wave in any medium (e.g. variations in the intensities of the local electric and magnetic fields in space, which constitutes electromagnetic radiation), there is a characteristic velocity at which the disturbance travels. For now we are mainly interested only in waves (photons) that travel at the velocity of light, but soon we will be asking about the wavelengths of particles that move much more slowly. There are three measurable properties of wave motion: amplitude, wavelength, and frequency, the number of vibrations per second (unit: Hertz = s-1). The relation between the wavelength λ (Greek lambda) and frequency of a wave (Greek nu) is determined by the propagation velocity v v=λ Example. What is the wavelength of the musical note A = 440 hz (=) when it is propagated through air in which the velocity of sound is 343 m s–1? λ = v/ = (343 m s–1)/(440 s–1) = 0.80 m = 80 cm. You should try the same using the velocity of light (c=3E10 cm s-1) at a frequency of 1 MHz or 1 GHz -- what wavelength regions do these correspond to? What is the frequency of a UV photon? A far infrared 100 micron photon? You should memorize the speed of light and be able to make these kinds of conversions easily. If you are not comfortable with the regions of the electromagnetic spectrum, now is the time to review. Here is a nice chart showing the names given to different wavelength ranges, their frequency, their energy, and what photons (or absorption of photons) are associated with different kinds of physical processes. Please make careful note of these processes and memorize the wavelengths and energies (in eV, or microns if appropriate) associated with each. If you find that you have to make a small copy of this illustration and glue it to your hand so that you can frequently study it, so be it. Two other attributes of waves are the amplitude (the height of the wave crests with respect to the base line) and the phase, which measures the position of a crest with respect to some fixed point. The square of the amplitude gives the intensity of the wave: the energy transmitted per unit time. We will see that Heisenberg took the bold step of representing a material particle as a wave, with the square of the amplitude (or wave function) interpreted as the probability of finding an electron in a certain region of space. A little thought will show you that the intensity (or flux, or irradiance, or whatever terminology might ring a bell) of light is no different, although it is usually expressed as an energy per unit something; normalize it to the total energy in the spectrum and it qualifies as the probability that the light will have a certain wavelength in a certain region of space. That is what we try to do when we solve the equation of radiative transfer. Back to waves: A unique property of waves is their ability to combine constructively or destructively, depending on the relative phases of the combining waves. In the early 19th century, the English scientist Thomas Young carried out the famous two-slit experiment which demonstrated that a beam of light, when split into two beams and then recombined, will show interference effects that can only be explained by assuming that light is a wavelike disturbance. The exact nature of the waves remained unclear until the 1860's when James Clerk Maxwell developed his electromagnetic theory. It was known that a moving electric charge gives rise to a magnetic field, and that a changing magnetic field can induce electric charges to move. Maxwell showed theoretically that when an electric charge is accelerated (by being made to oscillate within a piece of wire, for example), electrical energy will be lost, and an equivalent amount of energy is radiated into space, spreading out as a series of waves extending in all directions. What is "waving" in electromagnetic radiation? According to Maxwell, it is the strengths of the electric and magnetic fields as they travel through space. The two fields are oriented at right angles to each other and to the direction of travel. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. As the electric field changes, it induces a magnetic field, which then induces a new electric field, etc., allowing the wave to propagate itself through space These waves consist of periodic variations in the electrostatic and electromagnetic field strengths. These variations occur at right angles to each other. Each electrostatic component of the wave induces a magnetic component, which then creates a new electrostatic component, so that the wave, once formed, continues to propagate through space, essentially feeding on itself. In one of the most brilliant mathematical developments in the history of science, Maxwell expounded a detailed theory, and even showed that these waves should travel at about 3E10 cm s–1, a value which experimental observations had shown corresponded to the speed of light. In 1887, the German physicist demonstrated that an oscillating electric charge (in what was in essence the world's first radio transmitting antenna) actually does produce electromagnetic radiation just as Maxwell had predicted, and that these waves behave exactly like light. It is now understood that light is electromagnetic radiation that falls within a range of wavelengths that can be perceived by the eye. The entire electromagnetic spectrum runs from radio waves at the long-wavelength end, through heat, light, X-rays, and to gamma radiation. It is important that you be familiar with the names of wavelength regions (remembering that they are arbitrary--products of how we detect them or specific physical effects that they cause: you should think about this fact--in case somebody someday says “Why is the boundary between X-rays and UV where it is?”). Here is a smaller version of the plot given earlier: QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Quantum theory of light Certain aspects of the interaction between light and matter that were observed during the decade after 1890 proved rather troublesome. The relation between the temperature of an object and the peak wavelength emitted by it was established empirically by Wilhelm Wein in 1893. This put on a quantitative basis what everyone knows: the hotter the object, the "bluer" the light it emits. How can this be understood? Black body radiation All objects above the temperature of absolute zero emit electromagnetic radiation consisting of a broad range of wavelengths described by a distribution curve, its spectrum. An idealized model, a body that absorbs and emits perfectly, was finally solved by Planck, by making the radical assumption that light consists of particles called photons, each with energy h. This allowed him to avoid the problem with the classical Rayleigh-Jeans law for black body radiation which gives infinite amounts of energy (“the UV catastrophe”). The details of the derivation will not be given here-you just have to know it is a model for the continuum radiation and is pretty good for a lot of objects, although not good enough for quantitative calculations; a useful idea for a blackbody is an over with a small window by which some radiation can escape and be observed. And know that it was for the description of this problem that Planck invented Planck’s constant! This curve of intensity of light as a function of wavelength (or frequency) and temperature is known as the Planck, or blackbody, spectrum. Here is the full formula: If we look at Planck's law for small frequencies h<<kT, we find an expression that contains no factors h (Taylor series expansion of exponent) This is the Rayleigh-Jeans law as derived from classical physics. If we integrate these laws over all frequencies we find for Planck's law, and infinity for the Rayleigh-Jeans law. The result has been experimentally confirmed, and predates Planck's law. At ordinary temperatures this radiation is almost entirely in the infrared region of the spectrum, but as the temperature rises above about 2000K, more energy is emitted in the visible wavelength region and the object begins to glow, first with red light, and then shifting toward the blue as the temperature is increased. The sun is yellow for a good reason! (See below.) The way the peak of the Planck curve changes with temperature is quantified by Wien's Law: T (K) = 0.29 / max(cm), max(cm) = 0.29/ T(K) This is extremely useful and informative, and simple! These relations are useful not just for planets or stars, but even for rocks that are emitting at some temperature, and even microscopic dust grains (with some qualifications). On the other hand, they have nothing to do with radiation that is produced nonthermally (e.g. synchrotron radiation). Example: for the Sun we can measure the spectrum and find max = 5500 Å = 5.5 x 10-5 cm From this we can estimate the surface temperature of the Sun using Wien's Law (this will only give the approximately correct answer if the solar spectrum does indeed look like a Planck spectrum). T = 0.29/5.5 x 10-5 = 5200 K. Not bad (sun’s effective temperature is more like 5800 K), considering that the sun’s spectrum is not a Planck spectrum (i.e. not a black body). Most of the objects we’ll discuss in this class are roughly at room temperature T ~ 300 K In this case max(cm) = 0.29/T = 9.8 x 10-4 cm = 9.8 µm infrared This is why the continuum radiation from grains, aerosols, planets, protostellar disks, and much of the interstellar medium, is mostly in the infrared part of the spectrum. Cooler objects (like the outer planets and moons, or the outer parts of protoplanetary disks) will radiate at even longer, far infrared or submillimeter wavelengths. This is why the advent of infrared satellite missions like IRAS (1984), up to today’s Spitzer mission, are so important for star and planet formation. At submillimeter wavelengths (say 1000 microns), different types of instruments must be used, but at least they can be used from the ground. Submillimeter and infrared continuum and spectral line astronomy dominate the study of planetary science, exoplanets, and the search for extraterrestrial life. In the case of life, the reason is two-fold: 1. The basic problem is detecting the faint planet in the glare of its parent star, and this is optimized by observing where the planet puts out most of its energy, the infrared for a planet at the earth’s temperature (which is of greatest interest--temperatures where liquid water can exist most of the time). 2. There are several atmospheric gases (CO2, H2O, O2, and especially O3) that have strong infrared vibrational bands (we’ll go over vibrational spectroscopy later). Some (O3, CH4, N2O, ...) are considered possible biomarkers--we will discuss in full depth towards the end of the course. The other important relation to remember is the Stefan-Boltzmann law, which says that the energy emitted per unit time by any unit area (say a cm2) of a blackbody, call it F (for flux) is proportional to T4: F = T4 The constant of proportionality is called the Stefan-Boltzmann constant. (Think about how you can get the radius of an object from this relation if you know its absolute luminosity, using F = L/4R2.) These relations are derived by differentiating (Wien) and integrating (Stefan-Boltzmann) the complete Planck distribution given earlier, and displayed below. This type of radiation has two important characteristics. First, the spectrum is a continuous one, meaning that all wavelengths are emitted, although with intensities that vary smoothly with wavelength. The other curious property of black body radiation is that it is independent of the composition of the object; all that is important is the temperature. Black body radiation, like all electromagnetic radiation, must originate from oscillations of electric charges which in this case were assumed to be the electrons within the atoms of an object acting somewhat as miniature Hertzian oscillators. It was presumed that since all wavelengths seemed to be present in the continuous spectrum of a glowing body, these tiny oscillators could send or receive any portion of their total energy. However, all attempts to predict the actual shape of the emission spectrum of a glowing object on the basis of classical physical theory proved futile. Quantization and matter waves In 1900, the great German physicist Max Planck (who earlier in the same year had worked out an empirical formula giving the detailed shape of the black body emission spectrum) showed that the shape of the observed spectrum could be exactly predicted if the energies emitted or absorbed by each oscillator were restricted to integral values of h, where ("nu") is the frequency and h is a constant 6.626E–34 J s which we now know as Planck's Constant. (Planck’s headstone at his gravesite purportedly has nothing written on it besides his name and this number; an amusing game and effective way to see physics in a new perspective is to rank physicists according to whether they have named after them fundamental constants, classes of elementary particles (e.g. bosons), laws, effects, etc.) The allowable energies of each oscillator are quantized, but the emission spectrum of the body remains continuous because of differences in frequency among the uncountable numbers of oscillators it contains.This modification of classical theory, the first use of the quantum concept, was as unprecedented as it was simple, and it set the stage for the development of modern quantum physics. The photoelectric effect was the turning point in understanding the quantization of energy. (I’m assuming you know what that effect is--go to Wikipedia if you don’t.) Although the number of electrons ejected from the metal surface per second depends on the intensity of the light, as expected, the kinetic energies of these electrons (as determined by measuring the retarding potential needed to stop them) does not, and this was definitely not expected. Just as a more intense physical disturbance will produce higher energy waves on the surface of the ocean, it was supposed that a more intense light beam would confer greater energy on the photoelectrons. But what was found, to everyone's surprise, is that the photoelectron energy is controlled by the wavelength of the light, and that there is a critical wavelength below which no photoelectrons are emitted at all. Albert Einstein quickly saw that if the kinetic energy of the photoelectrons depends on the wavelength of the light, then so must its energy. Further, if Planck was correct in supposing that energy must be exchanged in packets restricted to certain values, then light must similarly be organized into energy packets. But a light ray consists of electric and magnetic fields that spread out in a uniform, continuous manner; how can a continuously-varying wave front exchange energy in discrete amounts? Einstein's answer was that the energy contained in each packet of the light must be concentrated into a tiny region of the wave front. This is tantamount to saying that light has the nature of a quantized particle whose energy is given by the product of Planck's constant and the frequency: E = h = hc/ where c is the speed of light. Einstein's publication of this explanation in 1905 led to the rapid acceptance of Planck's idea of energy quantization, which had not previously attracted much support from the physics community of the time. It is interesting to note, however, that this did not make Planck happy at all. Planck, ever the conservative, had been reluctant to accept that his own quantized-energy hypothesis was much more than an artifice to explain black-body radiation; to extend it to light seemed an absurdity that would negate the well-established electromagnetic theory and would set science back to the time before Maxwell. Einstein's special theory of relativity arose from his attempt to understand why the laws of physics that describe the current induced in a fixed conductor when a magnet moves past it are not formulated in the same way as the ones that describe the magnetic field produced by a moving conductor. The details of this development are not relevant to our immediate purpose, but some of the conclusions that this line of thinking led to very definitely are. Einstein showed that the velocity of light, unlike that of a material body, has the same value no matter what velocity the observer has. Further, the mass of any material object, which had previously been regarded as an absolute, is itself a function of the velocity of the body relative to that of the observer (hence "relativity"). As you probably know, according to Einstein, the mass of an object increases without limit as the velocity approaches that of light. Where does the increased mass come from? Einstein's answer was that the increased mass is that of the kinetic energy of the object; that is, energy itself has mass, so that mass and energy are equivalent according to the famous formula E = mc2. The only particle that can move at the velocity of light is the photon itself, due to its zero rest mass. In 1924, the French physicist Louis deBroglie proposed that, although the photon has no rest mass, its energy, given by E=h, confers upon it an effective mass of (since E=mc2) “m” = E/c2 = h/c2 = h/c and a momentum of “mv” = h/c2 x c = /c = h / or p = h/ = hk/2. DeBroglie (in his doctoral thesis) proposed that just as light possesses particle-like properties, so should particles of matter exhibit a wave-like character. Within two years this hypothesis had been confirmed experimentally by observing the diffraction (a wave interference effect) produced by a beam of electrons as they were scattered by the row of atoms at the surface of a metal. (Needless to say, deBroglie got a good postdoctoral position.) deBroglie showed that the wavelength of a particle is inversely proportional to its momentum: = h/m v where v is the velocity of the particle. Notice that the wavelength of a stationary particle is infinitely large, while that of a particle of large mass approaches zero. For most practical purposes, the only particle of interest to chemistry that is sufficiently small to exhibit wavelike behavior is the electron (mass 9.11E-28 g). I will some day ask you a question like: What is the deBroglie wavelength of a baseball travelling at 100 miles/hr? What is the deBroglie wavelength of an electron moving around an H atom in the ground state? What velocity should you use? A wave is a change that varies with location in a periodic, repeating way. What sort of a change do the crests and troughs of a "matter wave" trace out? The answer is that the wave amplitude at some position x represents the value of a quantity whose square is a measure of the probability of finding the particle at position x. In other words, what is "waving" is the value of a mathematical probability function. The evolution of this function is described by the Schrodinger equation. In 1927, Werner Heisenberg proposed that certain pairs of properties of a particle cannot simultaneously have exact values. In particular, the position and the momentum of a particle have associated with them uncertainties x and p given by (x) (p) ≥ h/2 As with the de Broglie particle wavelength, this has practical consequences only for electrons and other particles of very small mass. It is very important to understand that these "uncertainties" are not merely limitations related to experimental error or observational technique, but instead they express an underlying fact that Nature does not allow a particle to possess definite values of position and momentum at the same time. This principle (which would be better described by the term "indeterminacy" than "uncertainty") has been thoroughly verified and has far-reaching practical consequences which extend to chemical bonding and molecular structure. We will examine these later. The uncertainty principle is consistent with particle waves, because either one really implies the other. Consider the following two limiting cases: · A particle whose velocity is known to within a very small uncertainty will have a sharply-defined energy (because its kinetic energy is known) which can be represented by a probability wave having a single, sharply-defined frequency. A "monochromatic" wave of this kind must extend infinitely in space: QuickTi me™ a nd a TIFF (Uncompre ssed ) decomp resso r are need ed to se e th is p icture. But if the peaks of the wave represent locations at which the particle is most likely to manifest itself, we are forced to the conclusion that it can "be" virtually anywhere, since the number of such peaks is infinite! Now think of the opposite extreme: a particle whose location is closely known. Such a particle would be described by a short wavetrain having only a single peak, the smaller the uncertainty in position, the more narrow the peak. To help you see how waveforms of different wavelength combine, two such combinations are shown below: QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. It is apparent that as more waves of different frequency are mixed, the regions in which they add constructively diminish in extent. The extreme case would be a wavetrain in which destructive interference occurs at all locations except one, resulting in a single pulse: QuickT i me™ and a T IFF (Uncompressed) decompressor are needed t o see thi s pi cture. Is such a wave possible, and if so, what is its wavelength? Such a wave is possible, but only as the sum (interference) of other waves whose wavelengths are all slightly different. Each component wave possesses its own energy (momentum), and adds that value to the range of momenta carried by the particle, thus increasing the uncertainty p. In the extreme case of a quantum particle whose location is known exactly, the probability wavelet would have zero width which could be achieved only by combining waves of all wavelengths-- an infinite number of wavelengths, and thus an infinite range of momentum dp and thus kinetic energy. As Young’s double slit experiment and other evidence soon showed, photons have to be considered as both particles and waves, or one or the other depending on the experimental situation. This seems like a conundrum, leading to 100s of books, many of which you have probably seen at your local bookstore. This is unfortunate, because there is no “answer” to the question of wave-particle duality, and all the fun thought experiments (e.g. Schrodinger’s cat) are just that: fun. There is no problem here if you realize that the idea of something in terms of properties or attributes is simply a model of our interpretation of perceptions or experiments, and bears an extremely uncertain and even tenuous relation to what is “really” “out there,” if anything. There are some “things” that we will call electrons and photons and they can be given attributes for the purpose of calculations using our little models, but their “real” nature may have little to do with these models. Only a slight digression: The conflation or confusion of a model for the thing it represents is called “reification” (one of my favorite words), or “hypostatization.” The old Mr. Magoo cartoons were all about reification due to a cognitive organism walking around with extremely nearsighted vision, and we are probably doing it constantly in daily life, so we won’t worry about it further (although it is certainly a more important subject than quantum mechanics, contrary to what most physicists must say in order to rescue the objective nature of reality, without which physics would be a sham; you will see examples of this as we go through the course). If you are interested in evidence and arguments that infants produce conceptual models in the same way that science does, or that non-human organisms have a conceptual apparatus, or that human conceptual organization and categorization is highly variable (but with some interesting uniformities also), see me outside of class. For now, we resume with the reification of the world in order to have problems to solve and homework to give. The hydrogen spectral lines That atoms have electron energies with well-defined values was a result not of theory, but of the work of Balmer (a Swiss school teacher in 1885) and others to find a formula that relates the wavelengths of the known spectral lines of hydrogen in a simple way. Balmer’s formula was not based on theory, but worked, and he predicted a fifth line; later 35 lines were discovered, all having wavelengths fitting his formula, which we write today as 1/ = R [(1/m2) - (1/n2) ] wherer R is the “Rydberg constant” = 1.096...E5 cm-1 and for Balmer m=2. Soon it was found that replacing m with 1 (Lyman) or 3 (Paschen), other series of H lines could be accounted for. These series’, called Lyman, Balmer, Paschen, Brackett, and Pfund (up to m=5) cover the UV to the IR. (There are embarrassing 8mm home movies of Lyman trying to climb mountains to detect “his series” in the solar spectrum, requiring high altitude to reduce the UV attenuation; he was unfamiliar with the molecular absorbers in our atmosphere so did not know that he would need a mountain as high as our stratosphere for this purpose.) Nearly all astronomers are extremely familiar with the first two of these series’. (Lyman’s lines have come in mighty handy too, since the Hubble Space Telescope UV spectrometer was decommissioned; very distant galaxies at redshift around 3 can be observed by the Lyman break being redshifted into the visible, and so are called Lyman break galaxies). There is no limit to how large n can be: values in the hundreds have been observed, although doing so is very difficult because of the increasingly close spacing of successive levels as n becomes large. Atoms excited to very high values of n are said to be in Rydberg states. (See http://www.phys.ttu.edu/~gglab/rydberg_state.html) Attempts to adapt Balmer's formula to describe the spectra of atoms other than hydrogen generally failed, although certain lines of some of the spectra seemed to fit this same scheme, with the same value of R. Today we know those were the hydrogenic atoms and that quantum mechanics does not fare a lot better for non-hydrogenic atoms, although a few cases must be counted as successes. As n becomes larger, the spacing between neighboring levels diminishes and the discrete lines merge into a continuum. This can mean only one thing: the energy levels converge as n approaches infinity. This convergence limit corresponds to the energy required to completely remove the electron from the atom; it is the ionization energy. At energies in excess of this, the electron is no longer bound to the rest of the atom, which is now of course a positive ion. But an unbound system is not quantized; the kinetic energy of the ion and electron can now have any value in excess of the ionization energy. When such an ion and electron pair recombine to form a new atom, the light emitted will have a wavelength that falls in the continuum region of the spectrum. Spectroscopic observation of the convergence limit is an important method of measuring the ionization energies of atoms. The Bohr model emerges Rutherford's demonstration that the mass and the positive charge of the atom is mostly concentrated in a very tiny region called the nucleus forced the question of just how the electrons are disposed outside the nucleus. By analogy with the solar system, a planetary model was suggested: if the electrons were orbiting the nucleus, there would be a centrifugal force that could oppose the electrostatic attraction and thus keep the electrons from falling into the nucleus. This of course is similar to the way in which the centrifugal force produced by an orbiting planet exactly balances the force due to its gravitational attraction to the sun. Niels Bohr was born in the same year (1885) that Balmer published his formula for the line spectrum of hydrogen. Beginning in 1913, the brilliant Danish physicist published a series of papers that would ultimately derive Balmer's formula from first principles. Bohr's first task was to explain why the orbiting electron does not radiate energy as it moves around the nucleus. This energy loss, if it were to occur at all, would do so gradually and smoothly. But Planck had shown that black body radiation could only be explained if energy changes were limited to jumps instead of gradual changes. If this were a universal characteristic of energy- that is, if all energy changes were quantized, then very small changes in energy would be impossible, so that the electron would in effect be "locked in" to its orbit. From this, Bohr went on to propose that there are certain stable orbits in which the electron can exist without radiating and thus without falling into a "death spiral". This supposition was a daring one at the time because it was inconsistent with classical physics, and the theory which would eventually lend it support would not come along until the work of de Broglie and Heisenberg more than ten years later. Since Planck's quanta came in multiples of h, Bohr restricted his allowed orbits to those in which the product of the radius r and the momentum of the electron mv (which has the same units as h, J-s) are integral multiples of h: 2rmv = nh (n = 1,2,3, . .) Each orbit corresponds to a different energy, with the electron normally occupying the one having the lowest energy, which would be the innermost orbit of the hydrogen atom. Taking the lead from Einstein's explanation of the photoelectric effect, Bohr assumed that each spectral line emitted by an atom that has been excited by absorption of energy from an electrical discharge or a flame represents a change in energy given by E = h = hc/, the energy lost when the electron falls from a higher orbit (value of n) into a lower one. Finally, as a crowning triumph, Bohr derived an expression giving the radius of the nth orbit for the electron in hydrogen as rn = h2 n2/(4 me e2) Substitution of the observed values of the electron mass and electron charge into this equation yielded a value of 0.529E-8 cm for the radius of the first orbit, a value that corresponds to the radius of the hydrogen atom obtained experimentally from the kinetic theory of gases. Bohr was also able to derive a formula giving the value of the Rydberg constant, and thus in effect predict the entire emission spectrum of the hydrogen atom. His formula for the energy of level n in hydrogen was where 0 is the permittivity of free space (from Maxwell’s equations), and the appearance of h shows clearly that the formula is of quantum origin. We view the model as primitive and even ridiculous today, even though it does incorporate the idea of quantization of energies, but Bohr’s success gained him what few of us will ever have, our pictures on stamps: Here are some more pictures to remind you of the Bohr model and what it explained. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Problems, problems. There were two kinds of difficulties. First, there was the practical limitation that it only works for atoms that have one electron-- that is, for H, He+, Li2+, etc. The second problem was that Bohr was unable to provide any theoretical justification for his assumption that electrons in orbits described by the preceding equation would not lose energy by radiation (all accelerating charges must radiate--says who?). This reflects the fundamental underlying difficulty: because de Broglie's picture of matter waves would not come until a decade later, Bohr had to regard the electron as a classical particle traversing a definite orbital path. You can find some derivations and elaborations about the Bohr model here: http://www.nat.vu.nl/~wimu/Bohrtheory.html Once it became apparent that the electron must have a wavelike character, things began to fall into place. The possible states of an electron confined to a fixed space are in many ways analogous to the allowed states of a vibrating guitar string. These states are described as standing waves that must possess integral numbers of nodes. The states of vibration of the string are described by a series of integral numbers n = 1,2,... which we call the fundamental, first overtone, second overtone, etc. The energy of vibration is proportional to n2. Each mode of vibration contains one more complete wave than the one below it. In exactly the same way, the mathematical function that defines the probability of finding the electron at any given location within a confined space possesses n peaks and corresponds to states in which the energy is proportional to n2. The electron in a hydrogen atom is bound to the nucleus by its spherically symmetrical electrostatic charge, and should therefore exhibit a similar kind of wave behavior. This is most easily visualized in a two-dimensional cross section that corresponds to the conventional electron orbit. But if the particle picture is replaced by de Broglie's probability wave, this wave must follow a circular path, and- most important of all- its wavelength (and consequently its energy) is restricted to integral multiples n = 1,2,.. of the circumference 2r=n (n = 1, 2, 3, ...) for otherwise the wave would collapse owing to self-interference. That is, the energy of the electron must be quantized; what Bohr had taken as a daring but arbitrary assumption was now seen as a fundamental requirement. Indeed the above equation can be derived very simply by combining Bohr's quantum condition 2rmv = nh with the expression mv = h/ for the deBroglie wavelength of a particle. QuickTi me™ and a T IFF (Uncom pressed) decom pressor are needed to see t his pict ure. Viewing the electron as a standing-wave pattern also explains its failure to lose energy by radiating. Classical theory predicts that an accelerating electric charge will act as a radio transmitter; an electron traveling around a circular wire would certainly act in this way, and so would one rotating in an orbit around the nucleus. In a standing wave, however, the charge is distributed over space in a regular and unchanging way; there is no motion of the charge itself, and thus no radiation. Orbitals Because the classical view of an electron as a localizable particle is now seen to be untenable, so is the concept of a definite trajectory, or orbit. Instead, we now use the word orbital to describe the state of existence of an electron. An orbital is really no more than a mathematical function describing the standing wave that gives the probability of the electron manifesting itself at any given location in space. More commonly (and loosely) we use the word to describe the region of space occupied by an electron. Each kind of orbital is characterized by a set of quantum numbers n, l, and m. These relate, respectively, to the average distance of the electron from the nucleus, to the shape of the orbital, and to its orientation in space. There is also a spin quantum number. We will learn about these in some gory detail soon! Are electrons in orbitals moving? In its lowest state in the hydrogen atom (in which l=0) the electron has zero angular momentum, so electrons in s orbitals are not in motion. In orbitals for which l>0 the electron does have an effective angular momentum, and since the electron also has a definite rest mass me = 9.11E-28 g, it must possess an effective velocity. Its value can be estimated from the Uncertainty Principle; if the volume in which the electron is confined is about 10–10 m, then the uncertainty in its momentum is at least h/(1010) = 6.6E–19 g cm s–1, which implies a velocity of around 107 m s–1, or almost onetenth the velocity of light. The stronger the electrostatic force of attraction by the nucleus, the faster the effective electron velocity. In fact, the innermost electrons of the heavier elements have effective velocities so high that relativistic effects set in; that is, the effective mass of the electron significantly exceeds its rest mass. This has direct chemical effects; it is the cause, for example, of the low melting point of metallic mercury and of the Why does the electron not fall into the nucleus? The negatively-charged electron is attracted to the positive charge of the nucleus. What prevents it from falling in? This question can be answered in various ways at various levels. All start with the statement that the electron, being a quantum particle, has a dual character and cannot be treated solely by the laws of Newtonian mechanics. We saw above that in its wavelike guise, the electron exists as a standing wave which must circle the nucleus at a sufficient distance to allow at least one wavelength to fit on its circumference. This means that the smaller the radius of the circle, the shorter must be the wavelength of the electron, and thus the higher the energy. Thus it ends up "costing" the electron energy if it gets too close to the nucleus. The normal orbital radius represents the balance between the electrostatic force trying to pull the electron in, and what we might call the "confinement energy" that opposes the electrostatic energy. This confinement energy can be related to both the particle and wave character of the electron. If the electron as a particle were to approach the nucleus, the uncertainty in its position would become so small (owing to the very small volume of space close to the nucleus) that the momentum, and therefore the energy, would have to become very large. The electron would, in effect, be "kicked out" of the nuclear region by the confinement energy. The standing-wave patterns of an electron in a box can be calculated quite easily. For a spherical enclosure of diameter d, the energy is given by E = n2 h2/8med2 where n = 1,2,3,. Electron Spin Each electron in an atom has associated with it a magnetic field whose direction is quantized; there are only two possible values that point in opposite directions. We usually refer to these as "up" and "down", but the actual directions are parallel and antiparallel to the local magnetic field associated with the orbital motion of the electron. The term spin implies that this magnetic moment is produced by the electron charge as the electron rotates about its own axis. Although this conveys a vivid mental picture of the source of the magnetism, the electron is not an extended body and its rotation is meaningless. Electron spin has no classical counterpart; the magnetic moment is a consequence of relativistic shifts in local space and time due to the high effective velocity of the electron in the atom. This effect was predicted theoretically by P.A.M. Dirac in 1928. Bohr's theory worked; it completely explained the observed spectrum of the hydrogen atom, and this triumph would later win him a Nobel prize. The main weakness of the theory, as Bohr himself was the first to admit, is that it could offer no good explanation of why these special orbits immunized the electron from radiating its energy away. The only justification for the proposal, other than that it seems to work, comes from its analogy to certain aspects of the behavior of vibrating mechanical systems. Standing wave modes in a plucked string In order to produce a tone when plucked, a guitar string must be fixed at each end (that is, it must be a bound system) and must be under some tension. Only under these conditions will a transverse disturbance be countered by a restoring force (the string's tension) so as to set up a sustained vibration. Having the string tied down at both ends places a very important boundary condition on the motion: the only allowed modes of vibration are those whose wavelengths produce zero displacements at the bound ends of the string; if the string breaks or becomes unattached at one end, it becomes silent. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. In its lowest-energy mode of vibration there is a single wave whose point of maximum displacement is placed at the center of the string. In musical terms, this corresponds to the fundamental note to which the string is tuned; in terms of the theory of vibrations, it corresponds to a "quantum number" of 1. Higher modes, known as overtones (and in music, as octaves), contain 2, 3, 4 and more points of maximum displacement (antinodes) spaced evenly along the string, separated by points of zero displacement (nodes). These correspond to successively higher quantum numbers and higher energies. The vibrational states of the string are quantized in the sense that an integral number of antinodes must be present. Note again that this condition is imposed by the boundary condition that the ends of the string, being fixed in place, must be nodes. Because the locations of the nodes and antinodes do not change as the string vibrates, the vibrational patterns are known as standing waves. A similar kind of quantization occurs in other musical instruments; in each case the vibrations, whether of a stretched string, a column of air, or of a stretched membrane. Standing waves in the hydrogen atom The analogy with the atom can be seen by imagining a guitar string that has been closed into a circle. The circle is the electron orbit, and the boundary condition is that the waves must not interfere with themselves along the circle. This condition can only be met if the circumference of an orbit can exactly accommodate an integral number of wavelengths. Thus only certain discrete orbital radii and energies are allowed, as depicted in the two diagrams below. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Unbound states If a guitar string is plucked so harshly that it breaks, the restoring force and boundary conditions that restricted its motions to a few discrete harmonically related frequencies are suddenly absent; with no constraint on its movement, the string's mechanical energy is dissipated in a random way without musical effect. In the same way, if an atom absorbs so much energy that the electron is no longer bound to the nucleus, then the energy states of the atom are no longer quantized; instead of the line spectrum associated with discrete energy jumps, the spectrum degenerates into a continuum in which all possible electron energies are allowed. The energy at which the ionization continuum of an atom begins is easily observed spectroscopically, and serves as a simple method of experimentally measuring the energy with which the electron is bound to the atom. Physical significance of the wave function How can such a simple-looking expression contain within it the quantum-mechanical description of an electron in an atom— and thus, by extension, of all matter? The catch, as you may well suspect, lies in discovering the correct form of Ψ , which is known as the wave function. As this names suggests, the value of Ψ is a function of location in space relative to that of the proton which is the source of the binding force acting on the electron. As in any system composed of standing waves, certain boundary conditions must be applied, and these are also contained in Ψ; the major ones are that the value of must approach zero as the distance from the nucleus approaches infinity, and that the function be continuous. When the functional form of has been worked out, the Schrödinger equation is said to have been solved for a particular atomic system. The details of how this is done are only outlined in this course, but the consequences of doing so are extremely important to us. Once the form of is known, the allowed energies E of an atom can be predicted from the above equation. Soon after Schrödinger's proposal, his equation was solved for several atoms, and in each case the predicted energy levels agreed exactly with the observed spectra. Quick derivation of Schrodinger’s equation We give an “easy” derivation of Schrodinger’s equation here. It is the steady state (timeindependent) case that we want to solve, but I’ll include the time dependence at first and then take the limit where ∂/∂t = 0. You should try to do the derivation from scratch with no time dependence. Given the uncertainty principle, it is clear that all we can derive is an equation for the probability that the electron will have a certain position at a certain time. The probability density (pdf) of its position is P(x) such that P(x) gives the probability that the particle will be between x and x+dx; this is just the standard definition of probability density function. Schrodinger’s equation will be for a wave function whose square is this probability density, a function of position, and, in 3D spherical coordinates, a function of r, , . It will turn out that the solutions are spherical harmonics of various orders and that is why the orbitals, which are surfaces of constant probability density, have the forms they do. For example, one solution might have r increasing away from the nucleus, reaching a peak, then decreasing, and the angular solution, instead of being uniform in the angles (which would give a spherically symmetric orbital) being peaked at in and (say); then you can see how the orbital (surface of constant probability) will be a pair of blobshaped objects with a minimum between them. The standard example of a classical wave is the motion of a string. Typically a string can move up and down, and the standard solution to the wave equation can be positive as well as negative. Actually the square of the wave function is a possible choice for the probability (this is proportional to the intensity for radiation). Now we try to argue what wave equation describes the quantum analog of classical mechanics, i.e., quantum mechanics. The basic idea is to use the standard representation of a propagating plane wave, i.e. which is a wave propagating in the x direction with wavelength = 2/k and frequency = /(2). But we interpret this wave as a propagating beam of particles. If we define the probability as the square of the wave function, it is not very sensible to take the real part of the exponential: the probability would be an oscillating function of x for given t. If we take the complex function , however, the probability, defined as the absolute value squared, is a constant (A2 ) independent of x and t, which is very sensible for a beam of particles. Thus we conclude that the wave function (x,t) is complex, and the probability density is (x,t) 2. Using de Broglie's relation for p and also use E = h. One of the important goals of quantum mechanics is to generalise classical mechanics. We shall attempt to generalise the relation between momenta and energy, to the quantum realm. Notice that (just use the property of the differential of an exponential) Qu ic kTi me™ a nd a TIFF (Unc om pres se d) de co mp re ss or are n ee de d to s ee th is pi ctu re . Qui ck Ti me™and a TIF F (Uncompress ed)dec ompres sor are needed to s ee th i s pi c tu re. TIar Fe F ne (U Qe nco uided cm kT imtpro e e™ see sse d an t)dhisd e api com ct u rpre.e ssor TIar Fe F ne (U Qe nco uided cm kT imtpro e e™ see sse d an t)dhisd e api com ct u rpre.e ssor QuickTi me™ a nd a TIFF (Uncompre ssed ) decomp resso r are need ed to se e th is p icture. QuickTi me™ a nd a TIFF (Uncompre ssed ) decomp resso r are need ed to se e th is p icture. Using this we can guess a wave equation of the form Actually using the definition of energy when the problem includes a potential (for our case it will be the Coulomb potential) (when expressed in momenta, this quantity is usually called a "Hamiltonian") we find the time-dependent Schrödinger equation We will only spend limited time on this equation. Initially we are interested in the timeindependent Schrödinger equation, where the probability (x,t) 2 is independent of time. In order to reach this simplification, we find that (x,t) must have the form QuickTi me™ and a TIFF (Uncompressed) decompressor are needed to see this picture. If we substitute this in the time-dependent equation, we get (using the product rule for differentiation) QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Quick Time™and a TIFF(Uncompress ed)dec ompres sor are needed to s ee th is pic tu re. Taking away the common factor we have an equation for that no longer contains time, the time-indepndent Schrödinger equation QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. The corresponding solution to the time-dependent equation is the standing wave QuickTi me™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Notice that in deriving the wave equation we replaced the number or by a differential acting on the wave function. The energy (or rather the Hamiltonian) was replaced by an "operator", which when multiplied with the wave function gives a combination of derivatives of the wave function and function multiplying the wave function, symbolically written as QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. This appearance of operators (often denoted by hats) where we were used to see numbers is one of the key features of quantum mechanics. The boundary conditions on this equation, which we just state here, are that 1. (x) is a continuous function, and is single valued. 2. (x,t) 2dx must be finite, so that the normalized quantity QuickTi me™ a nd a TIFF (Uncompre ssed ) decomp resso r are need ed to se e th is p icture. is a probability density. 3. (x)/x is continuous except where V(x) has an infinite discontinuity (we will use this when solving the particle in the box problem). OK, now we see that there is an equation to solve but we will just presume we have the solutions. There is another very useful kind of information contained in . Recalling that its value depends on the location in space with respect to the nucleus of the atom, the square of this function ||2, evaluated at any given point, represents the probability of finding the electron at that particular point. Although the electron remains a particle having a definite charge and mass, and the question of "where" it is located is no longer meaningful. Any single experimental observation will reveal a definite location for the electron, but this will in itself have little significance; only a large number of such observations (similar to a series of multiple exposures of a photographic film) will yield meaningful results which will show that the electron can "be" anywhere with at least some degree of probability. This does not mean that the electron is "moving around" to all of these places, but that (in accord with the uncertainty principle) the concept of location has limited meaning for a particle as small as the electron. If we count only those locations in space at which the probability of the electron manifesting itself exceeds some arbitrary value, we find that the Ψ function defines a definite three-dimensional region which we call an orbital. The region near the nucleus can be thought of as an extremely small funnel-shaped box, the walls of which correspond to the electrostatic attraction that must be overcome if an electron confined within this region is to escape. As an electron is drawn toward the nucleus by electrostatic attraction, the volume to which it is confined diminishes rapidly. Because its location is now more precisely known, its kinetic energy must become more uncertain; the electron's kinetic energy rises more rapidly than its potential energy falls, so that it gets ejected back into one of its allowed values of n. The electron well. The red circles show the average distance of the electron from the nucleus for the allowed quantum levels (standing wave patterns) of n=1 through n=3. As n decreases the potential energy of the system becomes more negative and the electron becomes more confined in space. According to the uncertainty principle, this increases the momentum of the electron, and hence its kinetic energy. The latter acts as a kind of "confinement energy" that restores the electron to one of the allowed levels. We can also dispose of the question of why the orbiting electron does not radiate its kinetic energy away as it revolves around the nucleus. The Schrödinger equation completely discards any concept of a definite path or trajectory of a particle; what was formerly known as an "orbit" is now an "orbital", defined as the locations in space at which the probability of finding the electrons exceeds some arbitrary value. It should be noted that this wavelike character of the electron coexists with its possession of a momentum, and thus of an effective velocity, even though its motion does not imply the existence of a definite path or trajectory that we associate with a more massive particle. Orbitals (again) The modern view of atomic structure dismisses entirely the old but comfortable planetary view of electrons circling around the nucleus in fixed orbits. As so often happens in science, however, the old outmoded theory contains some elements of truth that are retained in the new theory. In particular, the old Bohr orbits still remain, albeit as spherical shells rather than as two-dimensional circles, but their physical significance is different: instead of defining the "paths" of the electrons, they merely indicate the locations in the space around the nucleus at which the probability of finding the electron has higher values. The electron retains its particle-like mass and momentum, but because the mass is so small, its wavelike properties dominate. The latter give rise to patterns of standing waves that define the possible states of the electron in the atom. The quantum numbers Modern quantum theory tells us that the various allowed states of existence of the electron in the hydrogen atom correspond to different standing wave patterns. In the preceding lesson we showed examples of standing waves that occur on a vibrating guitar string. The wave patterns of electrons in an atom are different in two important ways: 1. Instead of indicating displacement of a point on a vibrating string, the electron waves represent the probability that an electron will manifest itself (appear to be located) at any particular point in space. (Note carefully that this is not the same as saying that "the electron is smeared out in space"; at any given instant in time, it is either at a given point or it is not.) 2. The electron waves occupy all three dimensions of space, whereas guitar strings vibrate in only two dimensions. Aside from this, the similarities are striking. Each wave pattern is identified by an integer number n, which in the case of the atom is known as the principal quantum number. The value of n tells how many peaks of amplitude (antinodes) exist in that particular standing wave pattern; the more peaks there are, the higher the energy of the state. The three simplest orbitals of the hydrogen atom are depicted above in pseudo-3D, in cross-section, and as plots of probability (of finding the electron) as a function of distance from the nucleus. The average radius of the electron probability is shown by the blue circles or plots in the two columns on the right. These radii correspond exactly to those predicted by the Bohr model. Physical significance of n The potential energy of the electron is given by the formula QuickTime™ and a TIF F (Uncompressed) decompressor are needed to see this picture. in which e is the charge of the electron, m is its mass, h is Planck's constant, and n is the principal quantum number. The negative sign ensures that the potential energy is always negative. Notice that this energy in inversely proportional to the square of n, so that the energy rises toward zero as n becomes very large, but it can never exceed zero. This formula was actually part of Bohr's original theory (compare with the formula given above), and is still applicable to the hydrogen atom, although not to atoms containing two or more electrons. In the Bohr model, each value of n corresponded to an orbit of a different radius. The larger the orbital radius, the higher the potential energy of the electron; the inverse square relationship between electrostatic potential energy and distance is reflected in the inverse square relation between the energy and n in the above formula. Although the concept of a definite trajectory or orbit of the electron is no longer tenable, the same orbital radii that relate to the different values of n in Bohr's theory now have a new significance: they give the average distance of the electron from the nucleus. As you can see from the figure, the averaging process must encompass several probability peaks in the case of higher values of n. The spatial distribution of these probability maxima defines the particular orbital. This physical interpretation of the principal quantum number as an index of the average distance of the electron from the nucleus turns out to be extremely useful from a chemical standpoint, because it relates directly to the tendency of an atom to lose or gain electrons in chemical reactions. The angular momentum quantum number The electron wave functions that are derived from Schrödinger's theory are characterized by several quantum numbers. The first one, n, describes the nodal behavior of the probability distribution of the electron, and correlates with its potential energy and average distance from the nucleus as we have just described. The theory also predicts that orbitals having the same value of n can differ in shape and in their orientation in space. The quantum number l, known as the angular momentum quantum number, determines the shape of the orbital. (More precisely, l determines the number of angular nodes, that is, the number of regions of zero probability encountered in a 360° rotation around the center.) When l = 0, the orbital is spherical in shape. If l = 1, the orbital is elongated into something resembling a figure-8 shape, and higher values of l correspond to still more complicated shapes— but note that the number of peaks in the radial probability distributions (below) decreases with increasing l. The possible values that l can take are strictly limited by the value of the principal quantum number; l can be no greater than n – 1. This means that for n = 1, l can only have the single value zero which corresponds to a spherical orbital. For historical reasons, the orbitals corresponding to different values of l are designated by letters, starting with s for l = 0, p for l = 1, d for l = 2, and f for l = 3. The shapes and radial distributions of the orbitals corresponding to the three allowed values of l for the n = 3 level of hydrogen are shown above. Notice that the average orbital radius r decreases somewhat at higher values of l. The function relationship is given by QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. in which z is the nuclear charge of the atom, which of course is unity for hydrogen. The magnetic quantum number An s-orbital, corresponding to l = 0, is spherical in shape and therefore has no special directional properties. The probability cloud of a p orbital is aligned principally along an axis extending along any of the three directions of space. The additional quantum number m is required to specify the particular direction along which the orbital is aligned. "Direction in space" has no meaning in the absence of a force field that serves to establish a reference direction. For an isolated atom there is no such external field, and for this reason there is no distinction between the orbitals having different values of m. If the atom is placed in an external magnetic or electrostatic field, a coordinate system is established, and the orbitals having different values of m will split into slightly different energy levels. This effect was first seen in the case of a magnetic field, and this is the origin of the term magnetic quantum number. In chemistry, however, electrostatic fields are much more important for defining directions at the atomic level because it is through such fields that nearby atoms in a molecule interact with each other. The electrostatic field created when other atoms or ions come close to an atom can cause the energies of orbitals having different direction properties to split up into different energy levels; this is the origin of the colors seen in many inorganic salts of transition elements, such as the blue color of copper sulfate. The quantum number m can assume 2l + 1 values for each value of l, from –l through 0 to +l. When l = 0 the only possible value of m will also be zero, and for the p orbital (l = 1), m can be –1, 0, and +1. Higher values of l introduce more complicated orbital shapes which give rise to more possible orientations in space, and thus to more values of m. Electron spin and the exclusion principle Certain fundamental particles have associated with them a magnetic moment that can align itself in either of two directions with respect to an external magnetic field. The electron is one such particle, and the direction of its magnetic moment is called its spin. The mechanical analogy implied by the term spin is easy to visualize, but should not be taken literally. Physical rotation of an electron is meaningless. However, the coordinates of the electron's wave function can be rotated mathematically. Electron spin is basically a relativistic effect in which the electron's momentum distorts local space and time. It has no classical counterpart and thus cannot be visualized other than through mathematics. Today it is believed that for particles such as electrons that possess half-integral values of spin, no two of them can be in identical quantum states within the same system. The quantum state of a particle is defined by the values of its quantum numbers, so what this means is that no two electrons in the same atom can have the same set of quantum numbers. This is known as the Pauli exclusion principle, named after the German physicist Wolfgang Pauli (1900-1958, Nobel Prize 1945). In the last section of this reading I will argue that there would probably no complex chemistry without the exclusion principle. The best non-mathematical explanation of the exclusion principle that I have come across is Phil Fraundorf's Candle Dances and Atoms page at U. Missouri-St. Louis, which appears to be a version of an explanation given by Richard Feynmann. The exclusion principle was discovered empirically and was placed on a firm theoretical foundation by Pauli in 1925. A complete explanation requires some familiarity with quantum mechanics, so all we will say here is that if two electrons possess the same quantum numbers n, l, m and s (defined elsewhere, in case you are not familiar with them), the wave function that describes the state of existence of the two electrons together becomes zero, which means that this is an "impossible" situation. A given orbital is characterized by a fixed set of the quantum numbers n, l, and m. The electron spin itself constitutes a fourth quantum number s, which can take the two values +1 and –1. Thus a given orbital can contain two electrons having opposite spins, which "cancel out" to produce zero magnetic moment. Two such electrons in a single orbital are often referred to as an electron pair. You can find out more a little more detail on the Pauli exclusion principle from Wikipdedia or Hyperphysics.com. [Physics students: Have you ever seen it derived from first principles? Have you ever seen anything derived from “first principles”?] For a more rigorous look at quantum mechanics, there are many options, a book still probably being the best. Cheaper, and good, online accounts can be found at several sites. One is the 2005 course by Niels Walet, Univ. Manchester at http://walet.phy.umist.ac.uk/QM/LectureNotes/ which works from the beginning up to the easy potentials to scattering to the hydrogen atom. Concluding Discussion Given the basic features of quantum mechanics: What part of the hierarchy of chemical complexity really does depend on the existence of the quantum nature of the microscopic world? To what extent do the properties of mesoscopic objects (that’s what I’m calling everything from protoplanetary grains to biomacomolecules and supramolecular assemblies) depend on quantum effects? In particular, it is the hierarchical layering of structure from monomers, to polymers, through the stages of macromolecular structure, to supramolecular assemblies, that constitute not only biology but the entire macroscopic world (if you think about it a while). Here are some simple-minded ways to answer this, both problematic. There are others-if you have one, please let me know. First: I think it is clear that if energy levels and other microscopic properties (that we refer to as their mechanical analogues, like angular momentum) were not quantized, that is, discrete, the world would be a mess. If electrons could be at any energy possible, even with larger probability for some than others, there would be no identifiable units of chemical complexity, from atoms, molecules, polymers, ... This progression of hierarchical structure, perhaps most amazingly clear in the structure of proteins, but everywhere else too, owes the discreteness of its levels of complexity to the discreteness of the quantum world. I would like to know if you have come across other speculations about this question. You should think about how a lot of physics and macroscopic phenomena does not depend on this discreteness at all, but other mysterious features of the microscopic world that are beyond our understanding. As an example: The equations of fluid mechanics, also called hydrodynamics, or of magnetohydrodynamics (MHD), can be derived an easy way (conserve this in a volume, conserve that in a volume) that makes it clear that the momentum equation is only a form of Newton’s second law, etc., but the rigorous derivation (the one that really justifies why the pressure tensor can be written as an isotropic part and an anisotropic part that must satisfy certain criteria...) shows that it really depends entirely on the existence of the conservation properties of the Boltzmann equation, which itself is a conundrum because it is where an arrow of time suddenly appears in physics (Boltzmann committed suicide, partly over this problem). I would claim that nowhere in that rigorous derivation can you find a trace of quantum theory, even if you derive higher-order corrections to the equations of fluid flow (they are not exact). So not everything in the world depends on the discreteness of the microscopic world. But discreteness of chemical levels of organization seems to. A problem with this is whether or not physics actually derives the discreteness of the quantum world, or whether, frustrated by observations of hydrogen lines, Bohr stuck out his neck and made an outlandish suggestion only to fit the data; in fact there is some question whether he thought it was “real.” We will see the same thing below, concerning the exclusion principle. Perhaps you have studied special relativity and noticed the same sort of thing (Einstein wondering, “What if...” and then everything falling in place; or was it his wife that thought of that while she was correcting his math errors?) SECOND: I would say, and probably others would agree, that If it were not for the exclusion principle, the atoms of all elements would behave in the same way, and there would be no chemistry! This is of such direct importance for us that you should understand it clearly. As we have seen, the lowest-energy standing wave pattern the electron can assume in an atom corresponds to n=1, which describes the state of the single electron in hydrogen, and of the two electrons in helium. Since the quantum numbers m and l are zero for n=1, the pair of electrons in the helium orbital have the values (n, l, m, s) = (1,0,0,+1) and (1,0,0,– 1)— that is, they differ only in spin. These two sets of quantum numbers are the only ones that are possible for a n=1 orbital. The additional electrons in atoms beyond helium must go into higher-energy (n>1) orbitals. Electron wave patterns corresponding to these greater values of n are concentrated farther from the nucleus, with the result that these electrons are less tightly bound to the atom and are more accessible to interaction with the electrons of neighboring atoms, thus influencing their chemical behavior. If it were not for the Pauli exclusion principle, all the electrons of every element would be in the lowest-energy n=1 state, and the differences in the chemical behavior the different elements would be minimal. Chemistry would certainly be a simpler subject, but it would not be very interesting! In particular, there is virtually no chance that the kinds of bonds between different atomic orbitals that give rise to organic chemistry and life would exist without the Pauli principle. So at this level we can say specifically how the origin of life depends on quantum mechanics. But there is are less severe constraints imposed by quantum mechanics, and what we want to know is, given the Pauli principle and the existence of molecules, what is the importance of, say, the deBroglie wavelength by the time we get to something the size of a protein or nucleic acid or dust particle? See if you can guess the deBroglie wavelength of a macromolecule just by thinking about their speeds. Where does quantum mechanics play a role then, besides the two possibly profound instances of discrete states and the exclusion principle? Certainly in allowing only certain vibrational states and transitions in the monomers that make up the macromolecules, but this is at the molecular level where we expect such quantum effects. I will give you one example, which has to do with the mobility of electrons on linear biopolymers, in our next problem set (I will be outlining it in class--it is pretty elementary). See if you can think of any others yourself, for any kind of mesoscopic particle. If they are minimal, then can we conclude that all we have to do is treat the macromolecule like some complex collection of dipoles and induced dipoles, throw in whatever other intermolecular forces are at work, add up all the forces between all the molecular building blocks, and minimize the total energy to find, say, the lowest-energy folding conformation of a protein? People have been trying this for many years and now basically admit that it is not soluble, but does this have anything to do with quantum mechanics, or is it simply that it is a combinatorial nightmare, with so many possible ways to fold and rotate at each amino acid “hinge” that no computer in the near future will have the computational power to solve such a problem? We will look at this later in the course. If the complex properties and behaviors of organic macromolecules are not due fundamentally to quantum effects, yet the particles are too small to be part of the “macroscopic” world, then to what should we attribute these properties? To partially answer this, think about the properties of gravitational systems. The twobody problem can be solved, the three- and higher- body problems can’t (except with severe approximations) because they are so nonlinear that they introduce chaotic behavior and also complex resonance effects, but at least we can integrate the equations of motion on a computer and get an accurate answer. For a system with a billion gravitating particles, we can appeal to theories stolen from the atomic world, like statistical mechanics, kinetic theory, fluid mechanics. But this is at the “macroscopic” level. The case that is most like ours might be a star cluster, and indeed, the evolution of a globular cluster remains challenging even for the largest specialized computers. However a little thought will show you why globular clusters are not going to show any complex behavior like biomolecules do: The gravitational field has no dipoles, because it has only one “charge,” attractive. Think about what a huge difference the + - nature of the electrostatic interaction makes, from the structure of the atoms and molecules to the weak forces that dominate supramolecular aggregates. [You physics majors: Does the existence of two signs for the Coulomb force emerge in any way from quantum theory? At a fundamental level, from what does it derive? Is there something conserved for electromagnetic fields that is not conserved for gravitational fields? Do they exist in our theories only because it was obvious from experiments that there were two signs, so of course it had to emerge from the theory? At what point?] Yet we still have not really answered our question: So the polarity of the electric charge is a major difference, and that gives rise to molecular binding and the rest. Why do some mesoscopic entities behave like DNA or even lipids, while others behave like microscopic rocks--and are we sure about the latter? More to come.