Letter2
advertisement

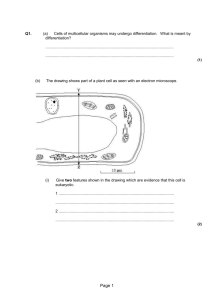
Catch Me If You Can? In the world we seem to be able to measure almost anything. We can measure the distances of galaxies, and the distance to the moon accurately. Even very small objects can be measured accurately. Light microscopes can magnify up to one thousand times. Transmission electron microscopes can magnify an amazing one hundred thousand times. The transmission electron microscope (TEM) operates on the same principles as the light microscope but uses electrons instead of light. What you can see with a light microscope is limited by the wavelength of light. TEMs use electrons as a "light source" and their much lower wavelength makes it possible to get a resolution a thousand times better than with a light microscope. These microscopes can view details in living cells and even objects close to atomic level. But what happens when you start to look closer. Why not a million times magnification or one hundred million times magnification? Even with the tiny wavelength of an electron compared to that of a light wave, it is still to large to view such small scales. But what if one were to have the perfect microscope? The world on very small scales, down to atomic level, gets very strange. Particles can no longer be viewed as sphere like objects, but rather a wave. It is hard to imagine a particle as a wave. This matter wave can be thought as a probability wave governing its position at a given time. The electron could be here at one time or there at another. It is more probable, for a hydrogen atom, for the electron to be in orbit than to have left the atom. But it is not impossible for this to occur. One can never know an electron’s exact position at one time but can give a probability of it being in a region. Just as it’s position cannot be exactly known, nor can its momentum. These two are linked in a very unique way, as discovered by Werner Heisenberg in 1927. The smaller you confine your region in space and start to pin point the electron, the less you will know about it’s momentum. But if you know more about the electron’s momentum you will know less about it’s position. These two uncertainties multiplied together give a constant. This constant is extremely small. This means the uncertainty principle governs the observable nature of atoms and subatomic particles while its effect in the macroscopic world is negligible and can usually be ignored. It is important to understand that this is part of the universe. It is not the fact that by measuring the position affects the momentum in anyway. With Heisenberg’s uncertainty principle, it can be shown that an electron in an atom will not exist inside the nucleus. By shrinking the volume of where an electron can be found to the size of the nucleus its energy exceeds a thousand times that of nuclear reactions. This makes it impossible for the electron to exist other than in an orbit, and shows what a remarkable principle this really is. 522 Words Aiden Dunne Dr.Bremmer