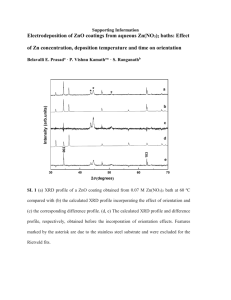
Supplementary material: XRD data for the fresh and aged PdCZ
advertisement
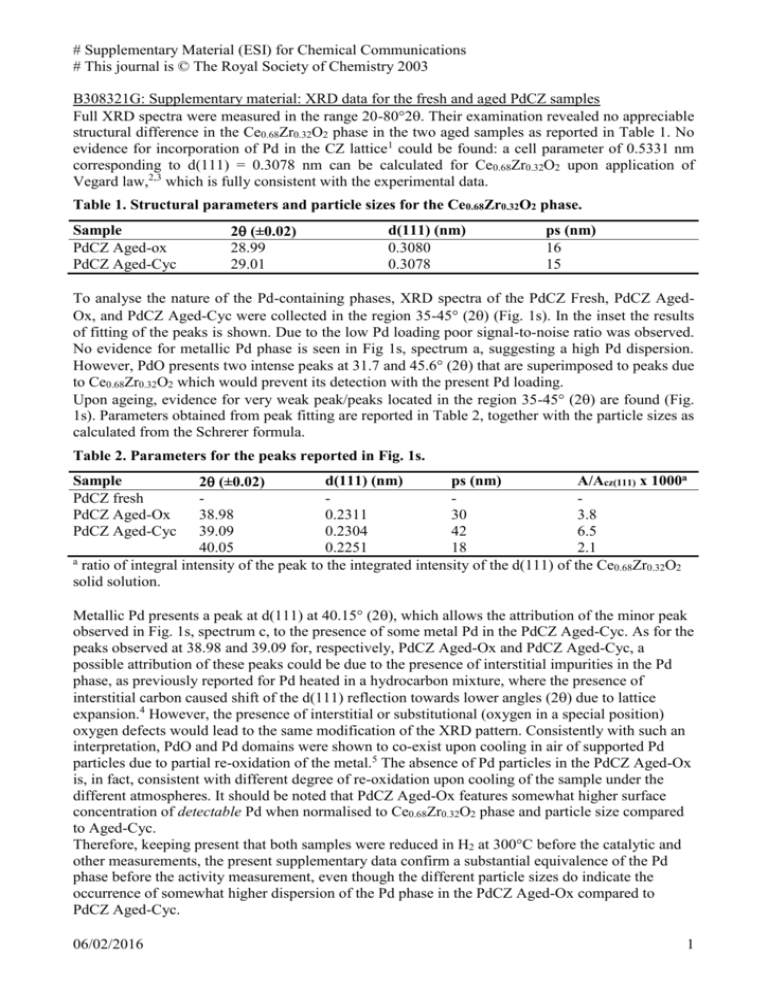
# Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 B308321G: Supplementary material: XRD data for the fresh and aged PdCZ samples Full XRD spectra were measured in the range 20-80°2. Their examination revealed no appreciable structural difference in the Ce0.68Zr0.32O2 phase in the two aged samples as reported in Table 1. No evidence for incorporation of Pd in the CZ lattice1 could be found: a cell parameter of 0.5331 nm corresponding to d(111) = 0.3078 nm can be calculated for Ce0.68Zr0.32O2 upon application of Vegard law,2,3 which is fully consistent with the experimental data. Table 1. Structural parameters and particle sizes for the Ce0.68Zr0.32O2 phase. Sample PdCZ Aged-ox PdCZ Aged-Cyc 2 (±0.02) 28.99 29.01 d(111) (nm) 0.3080 0.3078 ps (nm) 16 15 To analyse the nature of the Pd-containing phases, XRD spectra of the PdCZ Fresh, PdCZ AgedOx, and PdCZ Aged-Cyc were collected in the region 35-45° (2) (Fig. 1s). In the inset the results of fitting of the peaks is shown. Due to the low Pd loading poor signal-to-noise ratio was observed. No evidence for metallic Pd phase is seen in Fig 1s, spectrum a, suggesting a high Pd dispersion. However, PdO presents two intense peaks at 31.7 and 45.6° (2 that are superimposed to peaks due to Ce0.68Zr0.32O2 which would prevent its detection with the present Pd loading. Upon ageing, evidence for very weak peak/peaks located in the region 35-45° (2) are found (Fig. 1s). Parameters obtained from peak fitting are reported in Table 2, together with the particle sizes as calculated from the Schrerer formula. Table 2. Parameters for the peaks reported in Fig. 1s. d(111) (nm) ps (nm) A/Acz(111) x 1000a 2 (±0.02) 38.98 0.2311 30 3.8 39.09 0.2304 42 6.5 40.05 0.2251 18 2.1 a ratio of integral intensity of the peak to the integrated intensity of the d(111) of the Ce0.68Zr0.32O2 solid solution. Sample PdCZ fresh PdCZ Aged-Ox PdCZ Aged-Cyc Metallic Pd presents a peak at d(111) at 40.15° (2), which allows the attribution of the minor peak observed in Fig. 1s, spectrum c, to the presence of some metal Pd in the PdCZ Aged-Cyc. As for the peaks observed at 38.98 and 39.09 for, respectively, PdCZ Aged-Ox and PdCZ Aged-Cyc, a possible attribution of these peaks could be due to the presence of interstitial impurities in the Pd phase, as previously reported for Pd heated in a hydrocarbon mixture, where the presence of interstitial carbon caused shift of the d(111) reflection towards lower angles (2) due to lattice expansion.4 However, the presence of interstitial or substitutional (oxygen in a special position) oxygen defects would lead to the same modification of the XRD pattern. Consistently with such an interpretation, PdO and Pd domains were shown to co-exist upon cooling in air of supported Pd particles due to partial re-oxidation of the metal.5 The absence of Pd particles in the PdCZ Aged-Ox is, in fact, consistent with different degree of re-oxidation upon cooling of the sample under the different atmospheres. It should be noted that PdCZ Aged-Ox features somewhat higher surface concentration of detectable Pd when normalised to Ce0.68Zr0.32O2 phase and particle size compared to Aged-Cyc. Therefore, keeping present that both samples were reduced in H2 at 300°C before the catalytic and other measurements, the present supplementary data confirm a substantial equivalence of the Pd phase before the activity measurement, even though the different particle sizes do indicate the occurrence of somewhat higher dispersion of the Pd phase in the PdCZ Aged-Ox compared to PdCZ Aged-Cyc. 06/02/2016 1 # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 Fig. 1s. Powder XRD patterns of (a) PdCZ fresh, (b) PdCZ Aged-Ox and (c) PdCZ Aged-Cyc samples. 12 a 10 counts 8 6 4 2 0 35 36 37 38 39 40 41 42 43 44 45 3 b 2.5 counts 2 1.5 counts 1 0.5 0 37 38 39 40 41 42 2theta 35 36 37 38 39 40 41 42 43 44 45 5 4 c counts 3 2 1 0 counts 38 38.5 39 39.5 40 40.5 41 -1 -2 2theta 35 36 37 38 39 40 41 42 43 44 45 References 1. A. Primavera, A. Trovarelli, C. de Leitenburg, G. Dolcetti and J. Llorca, Stud. Surf. Sci. Catal. 1998, 119, 87. 2. M. Yashima, N. Ishizawa and M. Yoshimura, J. Amer. Ceram. Soc. 1992, 75, 1541. 3. M. Yashima, N. Ishizawa and M. Yoshimura, J. Amer. Ceram. Soc. 1992, 75, 1550. 4. S. B. Ziemecki and J. A. Jones, J. Catal. 1985, 95, 621. 5. A. K. Datye, J. Bravo, T. R. Nelson, P. Atanasova, M. Lyubovsky and L. Pfefferle, Appl. Catal. A Gen. 2000, 198, 179. 06/02/2016 2