Electronic supplementary information
advertisement

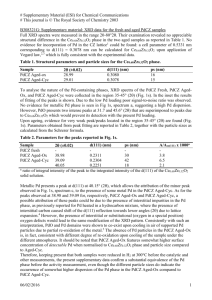
Electronic supplementary information (ESI) Template-free Synthesis of Nd0.1Bi0.9FeO3 Nanotubes with Large Inner Diameter and Wasp-Waisted Hysteresis Loop X. Li a, F. Guo a, S. Y. Wang a,b*, Xu Wang a, X.L. Xu c, J. Gao b, W.F. Liu c* E-mail: shouyu.wang@yahoo.com, & wfliu@tju.edu.cn I. Materials and Methods NBFO samples were synthesized using a sol-gel based electrospinning method. In a typical preparation process, bismuth nitrate (Bi(NO3)3·5H2O), neodymium nitride (Nd(NO3)3·6H2O) and iron nitrate (Fe(NO3)3·9H2O) were dissolved in DMF (N, N-dimethylformamide, C3H8O2) in stoichiometric proportions (9:1:10). After a period of stirring, polyvinyl pyrrolidone (PVP, Mw=1 300,000) was added to the settled solution. After being vigorously stirred by a magnetic stirrer for 12 h, the sol-gel solution was transferred into a dedicated electrospinning setup. The electrospinning process was performed at a voltage of 15 kV, with 10 cm spacing between the needle tip and collector, and a feed rate of 0.3 mL/h. The electro spun polymer composite nanofibers were collected using an alumina crucible and heated at 280 oC for 1 h firstly, and then were increased to the designed calcination temperature of 400-600 oC with a heating rate of 1 o C/min. At that specified temperature the as-spun NBFO NFs were calcined for 2 h in air, followed by a cooling procedure down to room temperature naturally in order to achieve good crystalline quality. II. Characterization The crystalline structure analysis of the samples was carried out by X-ray diffraction (XRD) (Rigaku D/MAX-2500 diffractometer with Cu Ka radiation). The morphology and size of the samples were investigated by scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) and the corresponding selected area electron diffraction (SAED) pattern using EM-2100F instrument. The phase purification, chemical bonding, and oxidation states of Bi, Fe, O, and Nd were analyzed by X-ray photoelectron spectrum (XPS), which was recorded with PHI1600. Magnetic characterization of samples was measured with Magnetic Property Measurement System (MPMS) SQUID-VSM made by Quantum Design. III. Experimental Results on XPS and XRD Phase purification, chemical bonding, and oxidation states of Bi, Fe, O, and Nd of NBFO NTs were analyzed by XPS through wide range of spectrum from 0 to 1100 eV and were shown in Fig. S3. There are XPS photoelectron peaks and the corresponding Auger lines of Bi, Fe, O, Nd and C elements. Insets of Fig. S3 show the narrow-scan spectra of Nd 4d (105-135 eV), Bi 4f (145-175 eV), Fe 2p (700-740 eV) and O 1s (515-540 eV) peaks for NBFO NTs. Herein, C1s peak located at 284.6 eV was used as the criterion to rectify the binding energy in XPS spectra. In addition to small amount of adsorbed carbon, no extra peaks of any impurity elements are detected in the spectrum up to 1100 eV, which is also confirmed by the XRD measurement. Fig. S4 shows the observed, calculated and difference in XRD patterns resulting from the Rietveld refinement of NBFO NTs. In XRD patterns, (104) and (110) diffraction peaks locating at around 2θ=32o have a tendency of merging into a single peak, as well as the other twin peaks, such as (006) and (202), (018) and (214), which is in contrary to rhombohedral bulk BFO with distinctly separated (104) and (110) peaks. Such characteristics agree well with our previous observation in Nd-doped BFO nanoparticles, and can be ascribed to a larger lattice distortion induced by the substitution of some Bi3+ ions with Nd3+ ions [1]. Structure parameters obtained from Rietveld refinement of NBFO samples are summarized in Table S1, and demonstrate a distorted rhombohedra structure with a space group of R3c of NBFO NTs. Some low-intensity peaks were detected around 2θ=27o for NTs sample, and can be identified as small amount of impurity phases Bi2Fe4O9. [1] H. Zhang, W. F. Liu, P. Wu, X. Hai, S.Y. Wang, G.Y. Liu, G.H. Rao, J. nanoparticle research, 16, 1, (2014). Fig. S1. SEM images of electrospun NBFO composite nanofibers after calcination at 500 o C (b) and 600 oC, insets showing the size distribution of diameters in the synthesized NBFO samples. Fig. S2 (a) TEM images of NBFO samples calcined at 500 oC; (b) shows the HRTEM image of front area in a single NBFO500 NFs; insets of (b) shows enlarged view of typical portion of NBFO500 samples and the corresponding SAED pattern of a single NFs. Fig. S3 Wide range of XPS spectrum from 0 to 1100 eV. Insets show the narrow-scan spectra of Nd 4d (105-135 eV), Bi 4f (145-175 eV), Fe 2p (700-740 eV) and O 1s (515-540 eV) peaks for the synthesized NBFO NTs. Fig. S4 Rietveld refined XRD pattern of different NBFO samples at room temperature. The intensities were normalized for plotting (a) 1.00 μm (b) 1.00 μm Fig. S1. SEM images of electrospun NBFO composite nanofibers after calcination at 500 o C (b) and 600 oC, insets showing the size distribution of diameters in the synthesized NBFO samples. (a) Fig. S2 (b) (a) TEM images of NBFO samples calcined at 500 oC; (b) shows the HRTEM image of front area in a single NBFO500 NFs; insets of (b) shows enlarged view of typical portion of NBFO500 samples and the corresponding SAED pattern of a single NFs. 110 700 Nd 4d Bi 4f 130 150 120 CKLM La 3d OKLM Bi 4p Fe 2p O 1s Fe LMM C 1s Bi 4d5/2 Bi 4d3/2 Bi 4f Bi 5d Fe 3p La 4d Intensity (a.u.) Fe 2p 720 740 O 1s 160 170 520 530 540 Nd4d 0 200 400 600 800 Binding Energy (eV) 1000 Fig. S3 Wide range of XPS spectrum from 0 to 1100 eV of NTs. Insets show the narrow-scan spectra of Nd 4d (105-135 eV), Bi 4f (145-175 eV), Fe 2p (700-740 eV) and O 1s (515-540 eV) peaks for the synthesized NBFO NTs. 600 C Intensity(a.u.) 550 C 500 C 450 C Yobs Ycal Yobs-Ycall Bragg position 400C 20 30 40 50 60 70 80 2 (degree) Fig. S4 Rietveld refined XRD pattern of different NBFO samples at room temperature. The intensities were normalized for plotting. Table S1. The structure parameters obtained from Rietveld refinement of NBFO samples. The unit cell structure of NBFO nanotubes could be considered as distorted perovskite with strongly distorted FeO6 octahedrons. Calcination Temp 400 oC 450 oC 500 oC 550 oC 600 oC Lattice parameters a=5.5702Å c=13.774Å V=370.14Å 3 a=5.5577Å c=13.683Å V=366.03Å 3 a=5.5644Å c=13.747Å V=368.62Å 3 a=5.5612 Å c=13.717Å V=367.39Å 3 a=5.5654 Å c=13.739Å V=368.54 Å 3 Bond angle Fe-O-Fe Rp Rwp χ2 155.038 o 8.5 11.9 2.13 156.090 o 8.1 11.5 1.90 155.037o 8.1 11.1 1.97 155.040o 8.1 11.4 2.10 155.039o 8.5 11.3 2.01