Atomic Spectra Worksheet: Chemistry Problems & Concepts
advertisement

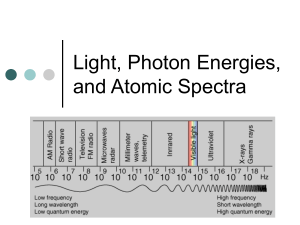
Chemistry Name ________________________________ Atomic Spectra Worksheet Date _____/_____/_____ Period _____ 1. How did Bohr expand on Rutherford’s model of the atom? The Rutherford model of the atom was incomplete because it didn’t explain how the atom’s negatively charged electrons were distributed in the space surrounding the positively charged nucleus. 2. Compare the energy of an electron in the ground state and an electron in the excited state. A valence electron in the ground state has to absorb energy in order for it to move or jump from the ground state to the excited state. This means that a ground state electron is lower energy than an excited state electron. 3. When an electron falls from a higher level to a lower level, how is the energy released? When a valence electron absorbs energy in the form of heat or light it uses that energy to jump to an excited state (outer level). An excited (valence) electron then releases that energy in the form of light (and a little heat) and falls back to its ground state. The movement of the valence electron from the ground state to the excited state is momentary. 4. Explain how the gaseous neon atoms in a neon sign emit light. Gaseous atom (nonmetal atoms) absorb energy in the form of high voltage electrical energy. The valence electrons of the gaseous atoms use this energy to jump from the ground state to an excited state and then emit (give off) that energy in the form of light (and a little heat). 5. List the seven colors of the visible spectrum in order of increasing energy. Red, Orange, Yellow, Green, Blue, Indigo, and Violet 6. What is the energy difference between a photon of yellow light and a photon of violet light? A photon of yellow light is lower energy than a photon of violet energy. 7. Determine the type of radiation (gamma rays, infrared waves, or radio waves) that has the: a. longest wavelength radio waves b. highest frequency gamma rays c. greatest energy gamma rays 8. Arrange the type of electromagnetic radiation (ultraviolet light, microwaves, radio waves, X-rays) in order of increasing: a. wavelength X–rays, ultraviolet light, microwaves, radio waves b. frequency radio waves, microwaves, ultraviolet light, X–rays c. energy radio waves, microwaves, ultraviolet light, X–rays 9. Compare the energy of the different types of radiation on the electromagnetic spectrum to help you answer the following questions. a. Why is ultraviolet (UV) radiation more harmful to your skin cells than visible light? (or …why is tanning dangerous?) UV light is higher energy than visible light and can damage or mutate skin cell. Long term exposure has the potential of causing skin cancer. b. You have to wear a lead shield when you get X-rays taken at the dentist. Why does lead shield the X-rays but will not block gamma radiation? Lead is a very dense (lead atoms are very close together) substance. Lead is too dense for x–rays to penetrate but not too dense for gamma rays to penetrate. 10. Compare the wave and particle models of light. Why is the Wave phenomena can only be explained by the particle model? The photoelectric effect (where wave energy from the sun is absorbed by a metal and turned into electrical energy) and the fact that waves slow down when they go through a different medium (like water) indicates that the wave must have some type of particle property. 11. What is the wavelength of electromagnetic radiation having a frequency of 5.00 x 1012 s-1? What kind of electromagnetic radiation is this? c = = 3.00 x 108 m/sec = (5.00 x 1012 Hz) = 6.00 x 10-5 m Radiation = Infrared Radiation 12. What is the frequency of electromagnetic radiation having a wavelength of 3.33 x 108 m? What type of electromagnetic radiation is this? c = = 3.00 x 108 m/sec = (3.33 x 108 m) = 9.01 x 10-1 Hz Radiation = Radio Waves 13. The laser in a CD player uses light with a wavelength of 7.70 x 10-7 m (780 nm). What is the frequency of this light? c = = 3.00 x 108 m/sec = (7.70 x 10-7 m) = 3.90 x 1014 Hz 14. A mercury lamp emits radiation with a wavelength of 4.36 x 10-7 m (436 nm). a. What is the color of the light from the mercury lamp? Violet b. Calculate the frequency of this radiation. c = = 3.00 x 108 m/sec = (4.36 x 10-7 m) = 6.88 x 1014 Hz 15. A very bright yellow line in the emission spectrum of sodium has a frequency of 5.10 x 1014 s-1. Calculate the wavelength of this yellow light. c = = 3.00 x 108 m/sec = (5.10 x 1014 Hz) = 5.88 x 10-7 m 16. When an electron falls from the forth to the second energy level, it emits a photon of green light with a frequency of 5.80 x 1014 s-1. Calculate the energy of this photon. E = h = (6.626 x 10-34 J ▪ sec)5.80 x 1014 Hz E = 3.84 x 10-19 J 17. A photon of red light has a wavelength of 6.45 x 10-7 m (645 nm). Calculate the energy of this photon. (hint: you will have to use both equations.) c = = 3.00 x 108 m/sec = (6.45 x 10-7 m) = 4.65 x 1014 Hz E = h = (6.626 x 10-34 J ▪ sec)4.65 x 1014 Hz E = 3.08 x 10-19 J 18. If it takes 8.17 x 10-19 J of energy to remove one electron from a gold surface. What is the wavelength of light capable of causing this effect? Is this wavelength of light part of the visible spectrum? (hint: you will have to use both equations.) E = h = (8.17 x 10-19 J) = (6.626 x 10-34 J ▪ sec) = 1.23 x 1015 Hz c = = 3.00 x 108 m/sec = (1.23 x 1015 Hz) = 2.44 x 10-7 m = 244 nm No it is not part of the visible spectrum.