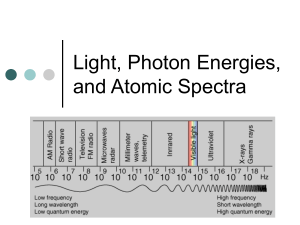
ATOMIC PHYSICS WORKSHEET
advertisement

MR. SURRETTE VAN NUYS HIGH SCHOOL CHAPTER 18: MODERN PHYSICS WORKSHEET SOLUTIONS 1. According to Einstein, what is true of the stopping potential for a photoelectric current as the wavelength of incident light becomes shorter? 1A. (E) increases (1) The maximum kinetic energy of photoelectrons increases as the frequency of incident photons increases: hf = Kmax + . (2) The speed of light is constant (c = 3.00 x 108 m/s). Therefore, frequency increases as wavelength decreases: c = f . 2. What is the de Broglie wavelength for a proton moving at a speed of 6.15 x 107 m/s? 2A. (1) p = mv (2) p = (1.67 x 10-27 kg)(6.15 x 107 m/s) (3) p = 1.03 x 10-19 kg.m/s (4) p = h / (5) = h / p (6) = (6.63 x 10-34 J.s) / (1.03 x 10-19 kg.m/s) (7) = 6.46 x 10-15 m 3. A nearby star’s surface temperature is 6200 K and the peak wavelength in its radiation is 475 nm. What is the surface temperature of a distant star where the peak wavelength is 585 nm? 3A. (1) T11 = T22 (2) T2 = (T11 / 2) (3) T2 = [(6200 K)(475 x 10-9 m) / (585 x 10-9 m)] (4) T2 = 5034 K 4. According to Einstein, increasing the brightness of a beam of light without changing its color will increase: 4A. (C) number of photons 5. How much energy (in eV) does a photon of light ( = 700 nm) have? 5A. (1) v = f (2) f = v / (3) f = (3.00 x 108 m/s) / (700 x 10-9 m) (4) f = 4.29 x 1014 Hz (5) E = hf (6) E = (4.14 x 10-15 eV.s)(4.29 x 1014 Hz) (7) E = 1.77 eV PHYSICS PAGE 1 MR. SURRETTE VAN NUYS HIGH SCHOOL 6. Light of wavelength 415 nm is incident onto the surface of a metal whose work function is 1.85 eV. 6a. What is the frequency of the light wave in petahertz? A. (1) v = f f = v / (3) f = (3.00 x 108 m/s) / 415 x 10-9 m (4) f = 7.23 x 1014 Hz (5) f = 0.723 PHz 6b. A. (1) (2) (3) Compute the photon energy in eV. E = hf E = (4.14 x 10-15 eV.s)(7.23 x 1014 Hz) E = 2.99 eV 6c. Will these photons eject electrons from the metal’s surface? A. Yes, the photon energy (2.99 eV) is greater than the minimum energy needed (1.85 eV), the work function(). 6d. A. (1) (2) (3) (4) What is the predicted value of the maximum kinetic energy of the photoelectrons? E = Kmax + Kmax = E – Kmax = 2.99 eV – 1.85 eV Kmax = 1.14 eV 6e. Would photons of wavelength 830 nm produce photoelectrons? A. No. Doubling the wavelength halves the photon energy: (1) v = f (2) f = v / (3) f = (3.00 x 108 m/s) / (830 x 10-9 m) (4) f = 3.61 x 1014 Hz (5) E = hf (6) E = (4.14 x 10-15 eV.s)(3.61 x 1014 Hz) (7) E = 1.50 eV Since the work function ( is 1.85 eV, photons of 1.50 eV will not release photoelectrons. PHYSICS PAGE 2 MR. SURRETTE VAN NUYS HIGH SCHOOL 7. If a hydrogen atom in the state n = 4 makes a transition to the state n = 2, what is the energy of the photon that is emitted? 7A. (1) E = (13.6 eV / (n2)2) – (13.6 eV / (n4)2) (2) E = (13.6 eV / 22) – (13.6 eV / 42) (3) E = 3.4 eV – 0.85 eV (4) E = 2.55 eV 8. The observed relativistic length of a super rocket moving by the observer at 0.875 c will be what factor times that of the measured rocket length at rest? 8A. (1) L = L’ [1 – v2/c2]1/2 (2) L = L’ [1 – (0.875 c)2/(1.0 c)2]1/2 (3) L = L’ (0.125)1/2 (4) L = (0.35) L’ (5) factor = 0.48 9. How fast would a rocket have to move past a ground observer if he observed a 12% length contraction in the rocket length? 9A. (1) L = L’ [1 – v2/c2]1/2 (2) (0.88 L’) = L’ [1 – v2/c2]1/2 (3) (0.88 L’) = L’ [1 – v2/c2]1/2 (4) 0.88 = (1 – v2/c2)1/2 (5) (0.88)2 = 1 – v2/c2 (6) - v2/c2 = (0.88)2 -1 (7) v2/c2 = 1 - (0.88)2 (8) v2/c2 = 0.0226 (9) v2 = (0.0226)(c2) (10) v2 = (0.0226)(3.00 x 108 m/s)2 (11) v = [(0.0226)(3.00 x 108 m/s)2]1/2 (12) v = 1.42 x 108 m/s 10. An unknown particle in an accelerator moving at a speed of 2.85 x 108 m/s has a measured relativistic mass of 3.2 x 10-26 kg. What is its rest mass? 10A. (1) m = mo / [1 – (v2/c2)]1/2 (2) mo = (m)[1 – (v2/c2)]1/2 (3) mo = (3.2 x 10-26 kg)[1 – (2.85 x 108 m/s)2 / (3.00 x 108 m/s)2]1/2 (4) mo = 1.6 x 10-27 kg PHYSICS PAGE 3 MR. SURRETTE VAN NUYS HIGH SCHOOL 11. An astronaut whose heart rate on Earth is 70 per minute increases his velocity to v = 0.980 c. What is his heart rate now as measured by an Earth observer? 11A. (1) t = (t)’ / [1 – (v2/c2)]1/2 (2) t =(t)’ / [1 - (0.980 c)2/(1.00 c)2]1/2 (3) t = (t)’ / 0.0199 (4) t’ = (0.0199) t (5) t’ = (0.0199)(70 per minute) (6) t’ = 13.9 per minute 12. The short lifetime of muons created in the upper atmosphere of the Earth would not allow them to reach the surface of the Earth unless their lifetime increased by time dilation. From the reference system of the muons, the muons can reach the surface of the Earth because: 12A. (D) length contraction decreases the distance to the Earth PHYSICS PAGE 4 MR. SURRETTE VAN NUYS HIGH SCHOOL CHAPTERS 17 - 18: THE ATOM, NUCLEUS, AND MODERN PHYSICS QUIZ SOLUTIONS 1. The restriction that no more than one electron may occupy a given quantum state was first stated by: 1A. Pauli 2. The equation E = hf was first formulated by: 2A. Planck 3. If the radius of the electron orbit in the n = 1 level of the hydrogen atom is 0.053 nm, what is the radius for the n = 2 level ? 3A. (1) rn = n2a0 (2) r2 = 22 (0.053 nm) (3) r2 = 2.12 x 10-10 m 4. A vapor lamp gives off light. Determine the wavelength of light given off if the energy difference Ef – Ei = 2.10 eV. 4A. (1) E = (2.10 eV)(1.6 x 10-19 J / 1 eV) (2) E = 3.36 x 10-19 J (3) E = hf (4) f = E / h (5) f = 3.36 x 10-19 J / 6.63 x 10-34 J.s (6) f = 5.07 x 1014 Hz (7) v = f (8) = v / f (9) = (3 x 108 m/s) / (5.07 x 1014 Hz) (10) = 592 nm 5. 12.1 eV is needed to ionize an unknown atom. From the ground state of an electron in a hydrogen atom, what wavelength is needed if a photon accomplishes this task? 5A. (1) E = (12.1 eV)(1.6 x 10-19 J / 1 eV) (2) E = 1.94 x 10-18 J (3) E = hf (4) f = E / h (5) f = 1.94 x 10-18 J / 6.63 x 10-34 J.s (6) f = 2.92 x 1015 Hz (7) v = f (8) = v / f (9) = (3 x 108 m/s) / (2.92 x 1015 Hz) (10) = 103 nm PHYSICS PAGE 5 MR. SURRETTE VAN NUYS HIGH SCHOOL 6. A radioactive material initially has an activity of 1200 counts/sec. If eight hours later it has an activity of 300 counts/sec, what is its half life? 6A. (1) 1200 600 300 (2) 2 half-lives have gone by, since each arrow represents one half-life. (3) Let x = one half-life (4) The total time is 8 hours (5) 2x = 8 hours (6) x = 4 hours 7. If there are 129 neutrons in X-205, how many neutrons are in X-208? 7A. (1) A1 = Z + N1 (2) Z = A1 – N1 (3) Z = 205 – 129 (4) Z = 76 (5) A2 = Z + N2 (6) N2 = A2 – Z (7) N2 = 208 – 76 (8) N2 = 132 8. What is the Q-value for the reaction 9Be + 12C + n? (m = 4.0026 u, mBe = 9.01218 u, mc = 12.0000 u, and mn = 1.008665 u) 8A. (1) mB = mBe + m mu4.0026 u (3) mB = 13.01478 u (4) mA = mc + mn (5) mA = 12.0000 u + 1.008665 u (6) mA = 13.008665 u (7) m = mB – mA (8) m = 0.006115 u (9) E = mc2 (10) E = (m)c2 (11) E = (0.006115 u)(931.5 MeV/u) (12) E = 5.69 MeV = Q-value 9. The energy released per fission event of U-235 is 195 MeV. This reaction is 37% efficient. Approximately how many fission events occur in one second to provide the 1.99 kW electrical power needs of a typical home? (1 eV = 1.6 x 10-19 J) PHYSICS PAGE 6 MR. SURRETTE VAN NUYS HIGH SCHOOL 9A. (1) Energy = (0.37)(195 x 106 eV) (2) Energy = 7.22 x 107 eV (3) 7.22 x 107 eV (1.6 x 10-19 J / 1 eV) (4) Energy = 1.15 x 10-11 J (5) Rate = (1990 J / 1 sec)(1 event / 1.15 x 10-11 J) (6) Rate = 1.73 x 1014 events / sec 10. How fast would a rocket have to move past a ground observer if he observed a 17% length contraction in the rocket length? 10A. (1) L = L’ [1 – v2/c2]1/2 (2) (0.83 L’) = L’ [1 – v2/c2]1/2 (3) (0.83 L’) = L’ [1 – v2/c2]1/2 (4) 0.83 = (1 – v2/c2)1/2 (5) (0.83)2 = 1 – v2/c2 (6) - v2/c2 = (0.83)2 -1 (7) v2/c2 = 1 - (0.83)2 (8) v2/c2 = 0.311 (9) v2 = (0.311)(c2) (10) v2 = (0.311)(3 x108 m/s)2 (11) v = [(0.311)(3x108 m/s)2]1/2 (12) v = 1.67 x 108 m/s 11. Light of wavelength 489 nm is incident on a metallic surface with a resultant photoelectric stopping potential (work function against an electron) of 0.8 V. What is the maximum kinetic energy of the emitted electrons? 11A. (1) v = f (2) c = f (3) f = c / (4) f = (3 x 108 m/s) / (489 x 10-9 m) (5) f = 6.13 x 1014 Hz (6) Kmax = hf – (7) hf = (6.63 x 10-34 J.s)(6.13 x 1014 Hz) (8) hf = 4.07 x 10-19 J (9) hf = 4.07 x 10-19 J (1 eV / 1.6 x 10-19 J) (10) hf = 2.54 eV (11) Kmax = 2.54 eV – 0.8 eV (12) Kmax = 1.74 eV PHYSICS PAGE 7 MR. SURRETTE VAN NUYS HIGH SCHOOL 12. An astronaut whose heart rate on Earth is 58 per minute increases his velocity to v = 0.899c. What is his heart rate now as measured by an Earth observer? 12A. (1) t = (t)’ / [1 – (v2/c2)]1/2 (2) t =(t)’ / [1-(0.899c)2/(1.00c)2]1/2 (3) t = (t)’ / 0.438 (4) t’ = 0.438 t (5) t’ = (0.438)(58 per minute) (6) t’ = 25.4 per minute 13. What is the wavelength (in nanometers) of a monochromatic light beam where the photon energy is 2.89 eV? 13A. (1) E= (2.89 eV) (1.6 x 10-19 J / eV) = 4.62 x 10-19 J (2) E = hf (3) f = E / h (4) f = (4.62 x 10-19 J) / (6.63 x 10-34 J.s) (5) f = 6.97 x 1014 Hz (6) v = f (7) c = f (8) = c / f (9) = (3 x 108 m/s) / (6.97 x 1014 Hz) (10) = 4.30 x 10-7 m (11) = 430 nm 14. What is the de Broglie wavelength for a particle (m = 3.21 x 10-26 kg) moving at a speed of 7.12 x 107 m/s? 14A. (1) p = mv (2) p = (3.21 x 10-26 kg)(7.12 x 107 m/s) (3) p = 2.29 x 10-18 kg.m/s (4) h = p (5) = h / p (6) = (6.63 x 10-34 J.s) / (2.29 x 10-18 kg.m/s) (7) = 2.90 x 10-16 m PHYSICS PAGE 8