Worked Solutions
advertisement
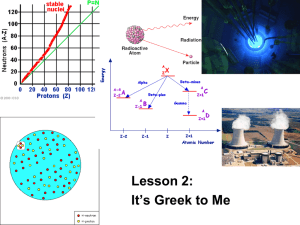
Worked Solutions Chapter 3 Question 16 Complete the following nuclear equations: (a) 238 4 U -- + He 2 92 Answer: When a 238 U isotope undergoes alpha decay, its atomic number decreases by 2, while its mass number decreases by 4: 238 U 92 (b) 234 Th 90 4 He 2 + 3 H -- 0 e -1 + 1 Answer: When a 3H isotope undergoes beta decay, its atomic number increases by 1, while its mass number remains the same: 3 H 1 3 (c) 239 Pu 0 He 2 -- + 4 He 2 + 93 Answer: When a 239 e -1 Pu isotope undergoes alpha decay, its atomic number decreases by 2, while its mass number decreases by 4: 239 235 Pa 91 Pu 93 (d) 4 He + 2 32 0 P -- + 15 e -1 Answer: When a 32P isotope undergoes beta decay, its atomic number increases by 1, while its mass number remains the same: 32 32 P 15 0 S 16 + e -1 1 (e) 212 Po 84 Answer: When a 212 -- 4 He 2 + Po isotope undergoes alpha decay, its atomic number decreases by 2, while its mass number decreases by 4: 212 Po 84 (f) 208 Pb 82 24 4 + 0 Na He 2 -- + e 11 -1 Answer: When a 24Na isotope undergoes beta decay, its atomic number increases by 1, while its mass number remains the same: 24 24 Mg 12 Na 11 (g) 226 0 + e -1 4 Ra -- + 88 He 2 Answer: When a 226 Ra isotope undergoes alpha decay, its atomic number decreases by 2, while its mass number decreases by 4: 226 Ra 88 (h) 222 4 Rn + He 86 2 131 0 I -- 53 + e -1 Answer: When an 131I isotope undergoes beta decay, its atomic number increases by 1, while its mass number remains the same: 131 131 I 53 0 Xe 54 + e -1 2 Question 19 The radioisotope cobalt-60 has a half-life of 5.26 years. How many years would it take 2g of cobalt-60 to decay to 0.25g? Answer: Since the half-life of cobalt-60 is 5.26 years, the original 2g would have halved to 1g after 5.26 years, the 1g would have halved to 0.5g after a further 5.26 years, and the 0.5g would have halved to 0.25g after a further 5.26 years. Thus it would take 15.78 years for 2g of cobalt-60 to decay to 0.25g. Question 31 (b) Complete the following nuclear equations: (i) 222 Rn 86 Answer: When a 222 -- 4 He 2 + Rn isotope undergoes alpha decay, its atomic number decreases by 2, while its mass number decreases by 4: 222 218 Po 84 Rn 86 (ii) 13 B 5 + 4 He 2 -- 0 + e -1 Answer: When a 13B isotope undergoes beta decay, its atomic number increases by 1, while its mass number remains the same: 13 B 5 (iii) 196 Au 79 Answer: When a 13 C 6 196 0 e + -1 -- + 4 He 2 Au isotope undergoes alpha decay, its atomic number decreases by 2, while its mass number decreases by 4: 3 196 Au 79 192 Ir 77 4 He 2 + (iv) 42 0 K -- + e 19 -1 Answer: When a 42K isotope undergoes beta decay, its atomic number increases by 1, while its mass number remains the same: 42 K 19 42 Ca 20 + 0 e -1 (v) 185 W 74 185 Answer: When a -- 4 He 2 + W isotope undergoes alpha decay, its atomic number decreases by 2, while its mass number decreases by 4: 185 W 75 181 Hf 72 + 4 He 2 (vi) 45 Ca 20 0 -- + e -1 Answer: When a 45Ca isotope undergoes beta decay, its atomic number increases by 1, while its mass number remains the same: 45 Ca 20 45 Sc 21 0 + e -1 (vii) 223 4 Ra 88 -- + He 2 4 Answer: When a 223 Ra isotope undergoes alpha decay, its atomic number decreases by 2, while its mass number decreases by 4: 223 219 Rn 86 Ra 88 4 He 2 + (viii) 112 0 Ag -- + e 47 -1 Answer: When a 112Ag isotope undergoes beta decay, its atomic number increases by 1, while its mass number remains the same: 112 Ag 112 Cd 47 + 48 0 e -1 (ix) 239 U 92 239 Np 93 + -- Answer: The atomic number of 239U increases by 1, while its mass number remains the same. This means that it has undergone beta decay: 239 U 92 239 Np 93 0 + e -1 (x) 232 Th 90 228 Ra + -- 88 Answer: The atomic number of 232Th decreases by 2, while its mass number decreases by 4. This means that it has undergone alpha decay: 232 Th 90 228 Ra 88 + 4 He 2 5