(U-238) decays into lead-206
advertisement

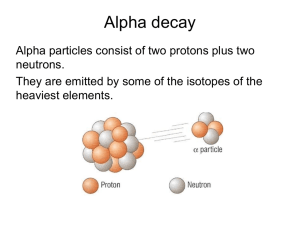
Radioisotopes, and their use in “dating” rocks Radioactive Decay Certain isotopes of some elements are not stable. They naturally change (decay) over time into other elements. For example: uranium-238 (U-238) decays into potassium-40 (K-40) decays into lead-206 (Pb-206) argon-40 (Ar-40) carbon-14 (C-14) nitrogen-14 (N-14) decays into Example – decay of uranium-235 into lead-207 Half-life = 700 million years (one half-life later) 50% remaining (two half-lives later) 25% remaining As the parent isotope decreases, the daughter isotope increases Daughter isotope percentage Parent isotope percentage Many steps in the decay of Uranium-238 into lead-206 Many steps in the decay of Uranium-238 into lead-206 (another way of looking at it) Many steps in the decay of Uranium-238 into lead-206 BUT – the first one determines the half-life (in this case) Symbol Element U-238 Uranium-238 Radiation alpha Th-234 Thorium-234 beta Pa-234 Protactinium-234 beta U-234 Uranium-234 alpha Th-230 Thorium-230 alpha Ra-226 Radium-226 alpha Rn-222 Radon-222 alpha Po-218 Polonium-218 alpha Pb-214 Lead-214 beta Bi-214 Bismuth-214 beta Po-214 Polonium-214 alpha Pb-210 Lead-210 beta Bi-210 Bismuth-210 beta Po-210 Polonium-210 alpha Pb-206 Lead-206 none Half-Life 4.46 billion years 24.1 days 1.17 minutes 247,000 years 80,000 years 1,602 years 3.82 days 3.05 minutes 27 minutes 19.7 minutes 1 microsecond 22.3 years 5.01 days 138.4 days stable Decay Product Th-234 Pa-234 U-234 Th-230 Ra-226 Rn-222 Po-218 Pb-214 Bi-214 Po-214 Pb-210 Bi-210 Po-210 Pb-206 (none) SO: U-238 has a half-life of 704 million years. Say a sample of a rock contains 800 atoms of the “parent” isotope (U-238), and only 200 atoms of the “daughter” isotope (Pb-206). How old is the rock? 800 atoms of the parent isotope, 200 atoms of the “daughter” isotope This means that 80% of the parent isotope remains, so about 0.4 half-lives have passed since the rock was formed (see the red line). Percentage of Parent Isotope Remaining Predicted Decay Rate of an Isotope over Time 100 90 80 70 60 50 40 30 20 10 0 0.4 x 704 million = 281 million years. 0 1 2 3 4 5 6 Number of Half-lives 7 8 9 10 Say a sample of a rock contains 250 atoms of the “parent” isotope (U-238), and 750 atoms of the “daughter” isotope (Pb-206). How old is the rock? (U-238 half-life = 704 million years) Percentage of Parent Isotope Remaining Predicted Decay Rate of an Isotope over Time 100 90 80 70 60 50 40 30 20 10 0 0 1 2 3 4 5 6 Number of Half-lives 7 8 9 10 This means that 25% of the parent isotope remains. so 2 half-lives have passed since the rock was formed. 2 x 704 million = 1408 million years (1.4 billion yrs) Percentage of Parent Isotope Remaining Predicted Decay Rate of an Isotope over Time 100 90 80 70 60 50 40 30 20 10 0 0 1 2 3 4 5 6 Number of Half-lives 7 8 9 10