Supplementary discussion
advertisement

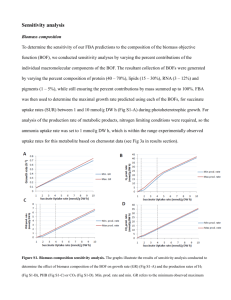
Supplementary Discussion “Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population” Jong Ah Kim, Christoffer Åberg, Anna Salvati, Kenneth A. Dawson Numerical Simulations of Nanoparticle Uptake by A Cell Population Undergoing Exponential Growth At all times, cells in the G2/M phase have had the longest time to take up nanoparticles, followed by cells in the S phase; in contrast, cells that have just divided are in the G1 phase. This fact is certainly consistent with the experimentally observed ranking (Fig. 1c), but in order to assess if this effect is quantitatively large enough to explain the experimental observation, simulations were performed with an ensemble of cells under somewhat idealised circumstances. In the simulations, all cells were assumed to cycle at the same rate. Different cycle lengths can be included, but would likely not lead to quantitatively different results. The positions of individual cells along the cell cycle, their ‘ages’, were randomly chosen according to an exponentially decaying distribution, as illustrated in the figure below. This distribution is well-known in cell proliferation analysis1,2,3, and leads to an exponentially growing population of cells. Exponentially decaying distribution along cell cycle with the individual phases indicated. Based on the distribution, the traversal time for a given phase can be related to the fraction of cells in that phase, as illustrated graphically. The shaded bins show a population of 106 cells randomly chosen according to the distribution and used as the initial population for the simulations. In the simulations, cells traversed the cell cycle with time. Since the exponentially decaying distribution (normalised for the total number of cells) is time-independent, the fraction of cells in each phase remains constant throughout the simulations. The time spent in each phase can then be related to the fraction of cells in a given phase2,3, as illustrated in the figure above. The fact that the fraction of cells in each phase remained constant in the simulations implicitly assumes that the cell population is in a state of exponential growth and that effects due to confluence have not been taken into account. We can therefore only compare the simulations with experiments for a limited time course, during which these assumptions remain approximately true. The simulations can be validated to data by using the nucleoside analogue EdU (5ethynyl-2’-deoxyuridine) together with DNA staining (7-AAD) to follow experimentally the division of those cells that were in S phase at the time of labelling. Since the labelled (S phase) cells have to traverse G2/M prior to division, a comparison with the simulations can be performed by counting the number of cells that have divided in the simulations, after a delay corresponding to the time spent in G2/M. The prediction, using only parameters independently measured (fraction of cells in the different phases) or acquired (cell population doubling time), is compared with the experimental data in Fig. 2b to excellent agreement. As each simulated cell traversed the cell cycle, it was also assumed to take up nanoparticles with a given rate. In the simplest case (Fig. 1e-f), the rate was assumed independent of the cell’s instantaneous position along the cell cycle. However, each cell was allowed to have its own rate, simulating a heterogeneity in the cell population when it comes to nanoparticle uptake. The rate with which an individual cell takes up nanoparticles was, furthermore, assumed to be inherited by both daughter cells upon cell division. A log-normal distribution of rates was used since it reproduces the appearance of the experimentally observed distributions measured by flow cytometry, though we imply no deeper significance than that. To investigate the effect of different rates of uptake during the different phases (Supplementary Fig. S17), simulations were performed where the uptake during a given phase was assumed constant, but different among the phases. Again each cell took up nanoparticles with its own rate; this rate being inherited by both daughter cells upon cell division. A 'fictitious rate', , was assigned to each cell from the same log-normal distribution as for the simpler case of constant rate during the cell cycle (previous paragraph). The rates of uptake during the different phases for a given cell, , and , respectively, were scaled from the 'fictitious rate' according to where denotes the measured rate and the fraction of cells in phase , respectively. The respective numerators in the expressions above imply that the ratios of the rates of uptake during the different phases of any given cell exactly match the ratios of the measured rates. In other words, a cell that takes up nanoparticles faster than average during the G0/G1 phase, also takes up nanoparticles faster than average during the S and G2/M phases. Furthermore, the rate of uptake for any given cell during G2/M phase is somewhat larger than during the other phases. The denominator in the above expressions was used for simplicity of comparison, since this normalisation ensures that the mean of the full population exactly matches that for the simpler case of constant rate of uptake during the different phases (previous paragraph). No export of nanoparticles was taken into account, and therefore the only diluting effect on the average number of internalised nanoparticles is cell division. Upon cell division, each simulated cell was in the simplest case (Fig. 1e-f and Supplementary Fig. S17) assumed to give exactly half of its intracellular load of nanoparticles to each daughter cell. To investigate the effect of asymmetrical partitioning in qualitative terms (Supplementary Fig. S9), simulations were also performed where one of the daughter cells received 70% and the other 30% of the intracellular load of the parent cell. In the numerical evaluation, we used a cell cycle length of 22 hours (the cell population doubling time given by the supplier), and fractions of cells in the different phases of 52% G0/G1, 40% S and 8% G2/M, respectively, as determined experimentally from the EdU and 7-ADD double staining (Supplementary Fig. S5). The initial population consisted of 106 cells (growing with time), but results were re-normalised to correspond to the 15,000 cells measured experimentally. The means corresponding to the different phases were calculated by averaging over the cells in a given phase, as defined by their position along the cell cycle (illustrated in the figure above). Results for the simplest case of equal rates of uptake during all phases and symmetrical split upon cell division are reported in Fig. 1: Figure 1f shows the average accumulations corresponding to the experiment shown in Fig. 1c, and Fig. 1e shows distributions of ‘cell fluorescence intensity’ corresponding to the experiment shown in Fig. 1d. Supplementary Fig. S9 shows the effect of asymmetrical partitioning, keeping the rates equal during the different phases, on the ‘cell fluorescence intensity’ distributions. Note that the average nanoparticle accumulation by cells instantaneously in a given phase is not affected by an asymmetrical partitioning, since the average partitioning is still half (for example, if one daughter receives 70% of the intracellular load and the other 30%, the average is still 50%). The averages corresponding to Fig. S9 are therefore the same as shown in Fig. 1f. In particular, this implies that the ranking of the phases is completely independent of eventual asymmetry of the partitioning. Supplementary Fig. S17 compares the average uptake with different rates of uptake in the different phases. Notably, the somewhat larger uptake rate measured during the G2/M phase (Fig. 3) does not affect the nanoparticle accumulation significantly, simply due to the fact that the time spent during G2/M is comparatively short. The observed ranking in nanoparticle accumulation for cells in the different phases is therefore most likely not due to the measured (minor) differences in uptake rates. In the absence of a nanoparticle source, the simulation predicts an exponential decay of the average number of nanoparticles per cell with a decay constant given by where is the cell population doubling time. Figure 2c shows the decay of the average fluorescence after loading cells with nanoparticles for some time, and subsequent removal of the nanoparticle source. A one-parameter fit (with initial fluorescence as a free parameter, and the decay constant fixed) shows that the simulation gives a good description of the experimental data (solid line in Fig. 2c). References 1. Gray, J. W. Cell cycle analysis from computer synthesis of deoxyribonucleic acid histograms. J. Histochem. Cytochem. 22, 642-650 (1974). 2. Steel, G. G. Growth Kinetics of Tumors: Cell Population Kinetics in Relation to the Growth and Treatment of Cancer (Oxford Univ. Press, Oxford, 1977). 3. Montalenti, F., Sena, G., Cappella, P. & Ubezio, P. Simulating cancer-cell kinetics after drug treatment: Application to cisplatin on ovarian carcinoma. Phys. Rev. E 57, 5877-5887 (1998).