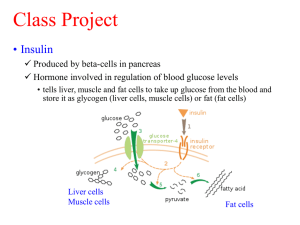
Checking the concentration of insulin:
advertisement

Checking the concentration of insulin: How accurate was your weighing out of the protein and then adding the correct amount of solution? We can check by using a spectrophotometer. Specs shine light (at a wavelength of 280 nm) on a solution of purified protein, and the protein’s aromatic amino acids (those with carbon rings attached to the amino acid; tryptophan) will absorb light at this wavelength. More tryptophan, more insulin, more absorbance of light. Problem is, some protein proteins do not have much tryptophan and you have to have a relatively pure sample of protein. This is a nondestructive method of measuring protein: other methods destroy the protein sample. The most common methods for finding the amount of protein are the BCA assay or the Bradford assay. In the BCA assay, proteins change Cu++ to Cu+ and this change in Cu increases the absorbance at 562 nm. The Bradford uses a dye (Coomassie blue G-250) which absorbs more light at 595 nm when protein is present. Bradford might be better because the absorbance does not change with incubation time. Problem with both: detergents screw up the assay (some companies claim new versions are detergent compatible). Proteins can be stored in a stock solution at -20 C in small aliquots (not a large volume like >2-3 mls but small volumes on the order of 500 microliters (500 µL, or half a milliliter). Dilutions of the stock solution are usually stable for months in the refrig. Sodium azide is sometimes added (0.02% or 20 mg per 100 ml) to keep bacteria from growing (watch out- this is a poison!). Absorbance of light of a certain wavelength = e (concentration) This is similar to the line equation we used before (y= mx + b) where the b term or y intercept is zero. “e” is the extinction coefficient and the slope of the line. The extinction coefficient is a constant for a given protein in a certain solution (the line between absorbance and concentration is linear, not curved, so the slope or extinction coefficient is constant). Someone somewhere already did our standard line for insulin: they placed various concentrations of insulin (using % solution not molarity for the concentration) in the spec and read out the absorbance at 280 nm. They found a slope or extinction coefficient of …. Turn on spectrophotometer, set the light wavelength to 276 (2,7,6, “goto wavelength”). Let the spect warm up for 15-30 min. Put a bit of your insulin solution in a quartz cuvette (little glass container you will use for your insulin solution) that does not absorb light at this low wavelength. DO NOT THROW away your insulin solution when done!! Read the optical density or OD (absorbance) of the carrier solution (solution without insulin in it): the 2 mM HCl solvent. Set the spec to zero. No insulin, you should see zero absorbance. Next, wash out the cuvette with a water spray bottle and put in the insulin solution; read the OD of the insulin solution. For example, OD276 = 0.642. Given the following: We know that a 0.1% insulin solution (0.1 g per 100 ml) has a certain absorbance and we know the extinction coefficient. How many grams of insulin are there in 1 ml of a 0.1% insulin solution? (answer in your lab notebook). OD at 276 for a 0.1% insulin solution is 1.05. What is the extinction coefficient for insulin? Record in your notebook. With the OD for your insulin solution, calculate the concentration of insulin: As a % solution: As the number of mg per 100 ml solution: As the number of mg per ml solution: As the number of g per Liter of solution: Divide the last g/L number by the molecular weight (insulin MW was noted above- the molecular weight is the number of grams per mole of insulin)-this gives you the insulin concentration in molarity (remember to use the symbol M underlined). Note that units cancel: g/L divided by grams/mole or g/L x mole/g = mole/L You need to check the calculation by using this method of unit cancellation. Express your answer in as a micromolar solution: _________ µM When we add the hormone to our cells, we want a final concentration of 1 or 2 µM so how much do you need of the insulin stock solution if you put your cells into 3 mls? For example: if your concentration is 100 µM insulin, then you need about 30 µL of insulin stock (practice; what if you got 145 µM insulin? How many µL would you add to 3 mls of cells?)