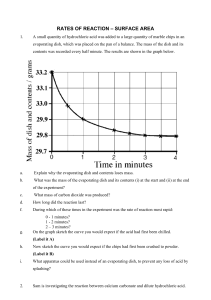
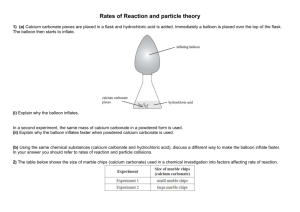
YEAR 12 PRACTICAL 2 - A
advertisement

YEAR 12 PRACTICAL 4 Determining the percentage purity of a sample of calcium carbonate In this experiment, you will dissolve a piece of impure calcium carbonate in excess hydrochloric acid. The calcium carbonate reacts with the acid as follows: CaCO3(s) + 2HCl(aq) CaCl2(aq) + CO2 + H2O(l) You will then titrate samples of 1.0 moldm-3 NaOH against the excess hydrochloric acid. Phase 1: Dissolving the calcium carbonate in excess hydrochloric acid 1. Weigh out accurately 3 – 4 g of impure calcium carbonate, and transfer it carefully into a 250 cm3 volumetric flask. 2. Carefully add 1.0 moldm-3 HCl to the volumetric flask until the calcium carbonate dissolves. 3. Then make the solution up to the 250 cm3 mark with 1.0 moldm-3 HCl and shake vigorously to ensure the contents are evenly mixed. Phase 2: Titrating the sodium hydroxide against the acid 1. Pipette 25.0 cm3 of 0.5 moldm-3 sodium hydroxide into a conical flask. 2. Add a few drops of phenolphthalein indicator. 3. Rinse the burette in the acid solution from the volumetric flask, and then fill the burette with the acid solution. 4. Titrate the alkali sample against the acid solution. 5. Repeat until 2 concordant values are obtained. 6. Record your titration results in a table. Phase 3: Determining the percentage purity of the calcium carbonate 1. Determine the number of moles of acid used in the titration. 2. Hence determine the number of moles of acid present in the 250 cm3 volumetric flask, after the reaction with calcium carbonate. 3. Calculate the moles of hydrochloric acid originally added to the volumetric flask, and hence the moles of hydrochloric acid used up in the reaction with calcium carbonate. 4. Hence determine the moles of calcium carbonate present, and the mass of calcium carbonate present. 5. Compare the mass of calcium carbonate with the mass of impure calcium carbonate weighed out at the start of the experiment, and hence calculate the percentage purity of the calcium carbonate.