CORPORATE FACT SHEET
CORPORATE PROFILE
Gamma Therapeutics, Inc., incorporated as a “C” corporation in Portland, Oregon, USA, October, 2009, is
an early stage biotechnology venture developing a novel class of biopharmaceutical and diagnostic test
solutions for the cardiovascular disease industry.
MISSION AND PURPOSE
Gamma Therapeutics, a product research and development company, will develop a host of new
commercial products to address the need for diagnostic testing and post-surgical therapy products in the
fast growing cardiovascular disease (CVD) market. The Company will develop its products from laboratory
to FDA regulatory approval and provide royalty-bearing non-exclusive and limited licenses for
manufacture, distribution and sales, to strategic business and channel sales partners.
FLAGSHIP PRODUCT
Gamma Therapeutics’ flagship product, GammaCoeur™ addresses the need for a new cardiovascular
disease risk assay to complement traditional diagnostic testing methods, such as those that determine
cholesterol, triglyceride and glucose levels. The GammaCoeur CVD Risk Assay will enhance cardiologists’
ability to assess heart attack and stroke risk by providing a new predictive and prognostic tool and assist
them in implementing early preventative care and treatment. This early intervention may help in reducing
the use and cost of long term drug regimens and invasive cardiac surgery, and help to improve the quality
of patient care and overall treatment outcomes.
TECHNOLOGY
Gamma Therapeutics product platform is based on a natural occurring clotting protein in human blood
called Gamma Prime Fibrinogen. Gamma Prime Fibrinogen is a carrier protein for factor XIII, a thrombin
binding site, forms clots that are resistant to fibrinolysis, and is specifically associated with cardiovascular
disease, (See Studies, 1, 2, and 3).
PRODUCTS
GammaCoeur™ A provisionally-patented (U.S.#61/314,134) measurable cardiovascular disease (CVD)
risk diagnostic assay to assist in prognosis and preventative care medicine.
GammaCoeur will be delivered as a lab-based, non-invasive, in vitro blood test.
Gammarin™
A patented (U.S. #7,615,527 B2) non-immunogenic, short acting anti-coagulant blood
thinner for post-surgical venous thromboembolism control. Gammarin will be delivered
intravenously as a biopharmaceutical drug replacing traditional blood thinner products.
GammaSeal™
A patent-pending, (U.S.Patent s/n) high strength surgical sealant for areas of mechanical
strain, i.e., large artery vascular surgery or wound repair. GammaSeal will be delivered
as a 2-part, fast clotting, sprayable sealant or drip.
MARKETS
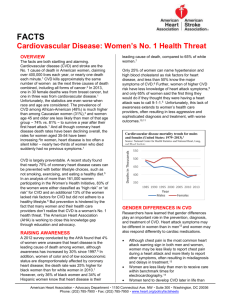
Cardiovascular disease (CVD), coronary heart disease and stroke are responsible for 16.7 million, or 29.9%
of total global deaths associated with some type of disease, according to the World Health Organization.
By 2010, CVD will be the leading cause of death in developing countries. In the U.S alone, $506 B is spent
annually on heart disease, including physician visits, treatment, drugs, diagnostics, hospitalization and
surgeries. Approximately $1.36 B of this total represents spending on diagnostic testing.
Cardiovascular disease includes a number of conditions affecting the structures or function of the heart.
•
Coronary artery (ischemic) heart disease (heart attack)
•
Abnormal heart arrhythmias
•
Heart failure
•
Heart valve disease
•
Congenital heart disease
•
Cardiomyopathy (heart muscle disease)
•
Pericardial disease
•
Marfan syndrome or aorta disease
•
Vascular disease
•
Rheumatic heart disease
Additional preventative methods to prevent heart attack is to determine the level of risk of the CVD
patient during check-ups or during times of patient distress that include diagnostic testing to assess
patient risk factors. Traditional risk factor tests, such as glucose, triglyceride and cholesterol levels,
provide cardiologists with the means to determine a prognosis and implement preventative care regimens.
Despite current diagnostic tests to assess heart disease or stroke risk, millions die each year from fatal
heart attacks, many of which were preventable with more predictive testing methods. Of the 500,000
reported heart attack deaths in the United States in 2009, 50% tested normal for cholesterol levels, often
considered the gold standard for heart risk assessment.
FUNDING
Gamma Therapeutics is currently funded with a $1.46 M, 3-year NIH Small Business Innovation Research
(SBIR) Grant to develop GammaCoeur. The company plans to build its first prototype assay in the Spring,
2011 and begin BETA testing in the Summer, 2011. Late 2012, early 2013 are target dates for FDA OIVD
approval.
STUDIES
(1)
Framingham Heart Study: Lovely et al. y' Fibrinogen: Evaluation of a New Assay for Study of
Associations with Cardiovascular Disease. Clinical Chemistry 2010; 56: 781-8
(2)
Stockholm Coronary Artery Risk Factor Study: Mannila et al. Elevated Plasma Fibrinogen y’
Concentration Increases Risk of Myocardial Infarction: Effects of Genetic Variation in Fibrinogen
Genes and Environmental Factors. Journal of Thrombosis and Haemostasis 2007; 5: 766-73
(3)
Penn State University CAD Study: Lovely et al. Association of y’A/y' Fibrinogen Levels and
Coronary Artery Disease. Thrombosis and Haemostasis 2002; 88: 26-31
WEBSITE
www.gamma-therapeutics.com
CORPORATE CONTACTS
David H. Farrell, PhD
Founder and Chief Scientific Officer
dfarrell@gamma-therapeutics.com
503.222.2314
David F. Eastman, MSc
Chief Executive Officer
deastman@gamma-therapeutics.com
503.222.2313
™GammaCoeur, GammaSeal, Gammarin, the product brand names and logos, and Gamma Therapeutics, the company name, letter
type and logomark, are trademarks of Gamma Therapeutics, Inc., Portland, Oregon. All Rights Reserved ©Copyright, 2010