PRES - This Is Not A Clinic
advertisement

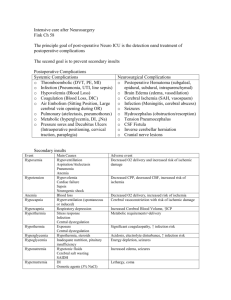
Posterior Reversible Encephalopathy Syndrome Introduction Posterior Reversible Encephalopathy Syndrome (PRES) is a radiological finding of non-specific etiology. PRES has been associated with many underlying etiologies but the primary pathological end-point is brain edema. History The term posterior reversible leukoencephalopathy syndrome was first used in 1996 by Hinchey et al. to describe a syndrome characterized by an association between the clinical symptoms of altered mental status, headache, seizures, blurred vision and the neuroimaging findings of posterior leukoencephalopathy 2. This name became a misnomer after it was observed that gray matter could also involved and the name was changed to posterior reversible encephalopathy syndrome 2. The syndrome has also been known by many names including occipital-parietal encephalopathy, reversible posterior leukoencephalopathy (RPLS), hyperperfusion encephalopathy, and brain capillary leak syndrome 1,3. Epidemiology There is no good data to available to give an estimate of the incidence of PRES 3. Age does not appear to be a risk factor as PRES has been reported in patients from age 2 to 90 years old 3. PRES has been associated with patients that have particular underlying clinical problems including 2: Hypertensive encephalopathy Preeclampsia/eclampsia/HELLP syndrome Immunosuppressive/cytotoxic medication use Renal disease Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome High-dose steroid therapy Liver failure Endocrine dysfunctions Electrolyte abnormalities Bone marrow transplantation Massive blood transfusion/EPO use Porphyria Various other causes Pathophysiology The basic mechanism of RPL is brain edema without infarction 1. There are three different theories as to the underlying cause of the brain edema. The first is the cytotoxic theory, and the second is the vasogenic theory 1. These two theories can be seen as two parts of a dichotomy. The third theory meanwhile belongs in a separate class and proposes that in some cases PRES can be caused by medications that damage the blood brain barrier. Cytotoxic Theory The precursor event of PRES in the cytotoxic theory is a sudden increase in blood pressure above what is “normal” for the patient 1. This sudden increase causes cerebral vascular damage and the vasculature reacts by vasoconstriction 1. It is this vasoconstriction that causes hypoxic damage to the brain parenchyma and therefore brain edema 1. Cerebral angiography in cases of PRES has shown vasoconstriction of the posterior and middle cerebral arteries which supports this theory 1. Yet, this vasoconstriction has not been seen consistently 1. The complete reversibility of radiographic changes with control of blood pressure is also not compatible with the cytotoxic edema resulting from vasospasm and ischemia proposed by this idea 1. Vasogenic Theory The vasogenic theory of PRES relies on our understanding of the autoregulation of cerebral vasculature. Autoregulation by the cerebral vessels is essential in maintaining cerebral perfusion. Cerebral autoregulation is effective when the mean arterial pressure (MAP) remains between 60-120 mmHg. The normal autoregulatory response to increasing MAP is cerebral vasoconstriction. A sudden rise in blood pressure can overcome the abilities of cerebral autoregulation to accommodate it 1. At a higher MAP instead of the normal response of vasoconstriction, there is arteriolar vasodilatation, endothelial dysfunction, capillary leakage and a disruption of the blood-brain barrier 1,2. This leads to extravasation within the extracellular space, particularly in the white matter, which is less tightly packed. The rate of elevation of blood pressure seems to be important and the cerebral vasculature of patients with chronic hypertension can withstand higher blood pressures without dysfunction than patients with acute increases in blood pressure from lower baseline levels 3. For those with chronic hypertension, blood pressures are usually required to be above 250/110 mm Hg to cause symptoms 4. It is curious that it is the posterior brain that is associated with PRES. The territory supplied by the posterior cerebral circulation may be more vulnerable to RPL because the posterior cerebral and vertebrobasilar arteries both have less sympathetic innervation than other parts of the cerebral circulation 1. This may translate into less capacity to withstand an acute increase in blood pressure by sympathetic mediated vasoconstriction 1. Evidence for the vasogenic theory comes from phase contrast angiography studies of flow in carotid and basilar arteries documenting an elevation in cerebral blood flow at high MAP, implying a breakdown of autoregulation 1. But the primary dysfunction in PRES is seen in the posterior cerebral areas. Light microscopy studies of RPL patients have shown edematous white matter consistent with vasogenic cerebral edema 1. Medication Induced Disruption of the Blood Brain Barrier A third proposal to account for some cases of PRES expounds on the role of the cytotoxic medications that these patients are sometimes taking. These drugs may have a direct toxic effect on the cerebral vascular endothelium that leads to endothelial dysfunction, capillary leakage and vasogenic edema 2. This theory is useful because not all cases of PRES have been associated with concurrent hypertension 2. PRES has also occurred in these patients even at “nontoxic” levels of these cytotoxic drugs 2. In a similar manner, other conditions such as sepsis, renal failure and electrolyte imbalance may predispose to RPL by first damaging the blood-brain barrier and thereby leading to vasogenic edema at MAP levels that would normally be within the range that is well tolerated 1. Clinical Presentation RPL characteristically presents as a headache, altered mental status, seizures and visual disturbances with or without accelerated hypertension 1. Multiple seizures are more common than single seizures 2. Visual problems may include hemianopsia, visual neglect or cortical blindness 2. An examination of the patient’s eyes is the most useful portion of the physical exam to confirm a suspicion of PRES 4. Ophthalmoscopy may reveal retinal arteriolar spasm, papilledema, retinal hemorrhages and retinal exudates 4. Diagnosis The major differential diagnosis of RPL is ischemic stroke 1. Differentiating between the two is essential since in RPL good blood pressure control is mandatory for treatment, whereas ischemic stroke is often treated with permissive hypertension 1. A complete differential diagnoses includes 2: CNS vasculitis o Granulomatous angiitis o Systemic lupus erythematous o Polyarteritis nodosa Acute or subacute neurological disease o Ischemic stroke o Progressive multifocal leukoencephalopathy o Acute disseminated encephalomyelitis o Infective encephalitis o Cerebral venous thrombosis o Cerebral autosomal dominant arteriopathy with stroke and ischemic leukoencephalopathy (CADASIL) Mitochondrial disease o Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) o Myoclonic epilepsy and ragged red fibers syndrome (MERRF) CNS collagen disease Hypoglycemia/hyponatremia Infectious etiologies Lumbar puncture A lumbar puncture is sometimes done because of the clinical presentation of headache and visual symptoms. An LP is generally not helpful but may show normal or elevated CSF pressure and protein 4. Imaging The diagnosis of RPL is made by imaging regardless of the underlying cause of PRES 2. MRI is both the favored modality and gold standard for diagnosis 1,2. Edema due to PRES is seen on an MRI as an increased signal on T2 and FLAIR in the occipital and posterior parietal lobes 1. The involved areas are usually bilateral and symmetrical 1. Fluid-attenuated inversion recovery (FLAIR) sequences should be performed when PRES is suspected because they significantly improve the ability to make the diagnosis 2. RPL lesions are seen particularly well on FLAIR because FLAIR nulls of the signal from ventricular and subarachnoid CSF which makes cerebral edema more prominent 1. Radiographic changes generally appear in the region of the posterior circulation 1. Grey matter is involved in 94% of cases 1. In a case series of 15 patients described by Hinchey et al. 60% had involvement of the anterior hemisphere 1. Other areas such as the brainstem, thalamus, and cerebellum may be involved in 56% of patients 1. Also noted has been a sparing of the calcerine fissure and paramedian occipital lobe structures 1. This can be used to distinguish RPL from a bilateral posterior cerebral artery infarction 1. Computed tomography can also be used to image PRES and may show areas of low attenuation in the posterior white matter 1. Treatment Blood Pressure Control Treatment of RPL primarily consists of early control of elevated blood pressure if present 1. Blood pressure control results in complete resolution of symptoms in most cases 1. The goal is a reduction of MAP by 20-25% within first 1-2 hours or a reduction of diastolic to 100mmHg 1. Blood pressure control is usually obtained using intravenous nicardipine, labetalol or nimodipine 2. Second line agents include sodium nitroprusside, hydralazine and diazoxide 2. Angiotensin converting anzyme (ACE) inhibitors are avoided in patients with hypovolemia, underlying renal artery stenosis or patients that are pregnancy 1. Treat of Underlying Cause Also essential is to treatment is the withdrawal or reduction of dosing of any medications that may have caused the RPL 1. Underlying renal failure should be addressed 1. Plasmapheresis may be indicated in patients with Thrombotic Thrombocytopenic Purpura 1. In pregnancy, delivery of the baby is the treatment of choice 1. Seizures The treatment of any accompanying seizures with done with anticonvulsants such as lorazepam or diazepam 2. The disappearance of seizures usually corresponds with the disappearance of radiographic abnormalities and long-term antiepileptic therapy is not required 1. Prognosis The term RPL has been stated to be a misnomer because the syndrome is not always reversible to the extent of full recovery even with adequate therapy 2. Also, changes in the brain are not necessarily localized to the posterior region of the brain and may also encompass changes in the areas supplied by the anterior and middle cerebral arteries 1. Involvement beyond the posterior circulation may a poor prognostic indicator and seen in more severe cases 1. Radiographic predictors of poor outcome include gadolinium enhancement, hemorrhage and infarction 1. Also, extensive abnormalities seen in T2 are predictive of worse prognosis 1. As a general rule, seizures disappear with radiographic abnormalities 1. There have been case reports of hypertensive encephalopathy later followed by chronic epilepsy 2 . Usually though, complete neurological recovery can be expected in most patients within 2 weeks 1. References 1. Stott V.L. Reversible Posterior Leukoencephalopathy Syndrome: A Misnomer Reviewed. Internal Medicine Journal 2005; 35:83-90 2. Servillo G. Posterior Reversible Encephalopathy Syndrome in Internsive Care Medicine. Intensive Care Med (2007) 33:230-236 3. Neill, Terry. Reversible Posterior Leukoencephalopathy Syndrome. UpToDate. Sept 17, 2007. 4. Aminoff, Michael. Clinical Neurology 6th Edition. Lange 2005.