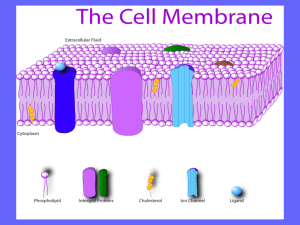
Chapter 1 – Introduction - eCommons@Cornell
advertisement

ANALYSIS OF WHOLE BLOOD SAMPLES: OPTIMIZATION OF SAMPLE PREPARATION FOR RAPID ASSAYS. A thesis presented to the faculty of the Graduate School of Cornell University in partial fulfillment of the requirements of the degree of Master of Engineering by Jocelyn J. Tan August 2008 © Jocelyn J. Tan, 2008 i Abstract Whole blood separation is a crucial step prior to the analysis of a whole blood sample in a lateral flow assay (LFA). While there are numerous diagnostic applications that utilize the technique for rapid and sensitive analysis, few are able to directly work with human whole blood samples and provide accurate and consistent results. The main objective of this project was to find and optimize a whole blood filter capable of effective whole blood filtration and plasma collection for the analysis of whole blood samples in the LFA format. The work focused on finding suitable filter materials that can be incorporated into any test strip in an LFA format, and explore the modifications required to enhance the filter membrane’s performance. Membranes examined for this purpose were polyvinylpyrrolidone (PVP)/ polyethersulfone (PES) Primecare membranes (Spectral Diagnostics), FUSION 5 (Whatman), and HanoRapid (Hanomy LLC.) Various factors evaluated included plasma yield, absorbance capacity, extent of hemolysis, leakage of red blood cells, unspecific binding, and wicking time. Based on the results, it was determined that the PVP/PES membrane X and NX, which separate and collect plasma from whole blood through a porosity gradient, were the most suitable membrane materials to be incorporated into the lateral flow strip test as whole blood filtering sample pads. By using the combination of X and NX as the sample pad for a lateral flow assay, diluted whole blood (95%) in PBS up to 30 µL spiked with insulin was filtrated and analyzed correctly within 30 minutes. Other work done in this project included improving the sensitivity of a lateral flow assay on HanoRapid nitrocellulose membranes for the detection of serum insulin through ii optimization of blocking solution. By blocking the membrane with a solution containing 0.03% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002% Tween20, the improved limit of detection for insulin on HanoRapid nitrocellulose membrane was achieved at 0.75 µg mL-1, which was still above the serum insulin concentration. Based on these results, it was concluded that a traditional lateral flow assay with HanoRapid membranes is not sensitive enough for the detection of serum insulin. iii Acknowledgements I would like to thank my advisor, Professor Antje Baeumner, for all of the support and help she has given me in completing this project, and for comforting me during my most difficult times. Also, Dr. Katie Edwards and Dr. Kit Meyers, who taught me everything I know about laboratory equipment and procedures, and Professor Daniel Aneshansley, who encouraged me to pursue this M.Eng degree. My thanks to the Department of Biological and Environmental Engineering at Cornell University, for making my experience at Cornell, both as an undergraduate and graduate student, enriching and memorable. I would also like to thank Nanogen Inc.(formerly Spectral Diagnostics Inc.) and Whatman for supplying PVP/PES filters and FUSION 5 samples. Lastly, thanks to my parents and friends, who have supported me and my academic interests, always. iv Biography Jocelyn Tan is from Guangzhou, China, where she has lived until the age of 15. She then moved to New York City and attended high school there. She pursued her Bachelor of Science and Master of Engineering degree in Biological Engineering at Cornell University, Ithaca, NY. v List of Figures Figure 2.1 Schematic of the lateral flow assay used in this study. ……………………4 Figure 3.1 A typical Millipore Hi-Flow Plus 120 membrane card with PVP/PES membranes provided by Spectral Diagnostics. ….……………..………….16 Figure 4.1 Lateral flow assays -1 for detection of 1xPBS buffered insulin….….…......20 Figure 4.2 Lateral flow assays- 2 for detection of 1xPBS buffered insulin…....………21 Figure 4.3 Lateral flow assays-3 for detection of 1xPBS buffered insulin …………....14 Figure 4.4 Lateral flow assays on FUSION 5 membranes strips ………………….…..24 Figure 4.5 Lateral flow assay on Millipore Hi-Flow plus 120 membrane cards with PVP/PES membranes (X/CS) ………………………………….………….26 Figure 4.6 Lateral flow assay results on Millipore Hi-Flow Plus 120 using the PVP/PES blood filtering membrane X from Spectral Diagnostics. ……………..…...27 Figure 4.7 Lateral flow assay results on Millipore Hi-Flow Plus 120 using the PVP/PES blood filtering membrane NX, C/Q, CS………………………………….. 28 Figure 4.8 Blood filtration/migration on Hanorapid membranes ……………...…….. 31 Figure 4.9 Filtration of whole blood (25 µL) spiked with PBS-buffered insulin …......31 Figure 4.10 Blood filtration/ migration on FUSION 5…………………………………33 Figure 4.11 Plasma Capillarity Diagrams on NX, CS, and C/Q-I ………..……………35 Figure 4.12 Whole blood filtration on Millipore Hi-Flow Plus 120 membrane using X(2cm) plus NX(2cm) as the sample pad………………………………….37 Figure 4.13 Whole blood filtration on Millipore Hi-Flow Plus 120 membrane using X(2cm) plus CS (2 cm) as the sample pad…………………………………38 vi Figure 4.14 Whole blood filtration on Millipore Hi-Flow Plus 120 membrane using X(2cm) plus CQ-I(2cm) as the sample pad………………………………..38 Figure 4.15 Filtration and analysis of 50% diluted whole blood spiked with insulin at 5µg/mL (30µL total)…………………………………………………..…...40 Figure 4.16 Filtration and analysis of 30µL of 50% diluted whole blood spiked with 5 µg/mL insulin ……………………………………………………………41 Figure 4.17 Filtration and analysis of 95% diluted whole blood in PBS ………………42 Figure 4.18 Filtration and analysis of 30 µL of 95% diluted whole blood in PBS spiked with insulin…………………………………...……………………............44 Figure 4.19 Filtration and analysis of 30 µL of 95% diluted whole blood spiked with insulin………………………………………………………………………44 Figure 4.20 Filtration and analysis of 30 µL 95% diluted whole blood spiked with insulin………………………………………………………………………46 vii List of Tables Table 3.1 Important membrane specifications…………………….…………..…………9 Table 3.2 Blocking solutions used with HanoRapid membranes…………………….....10 Table 3.3 Important membrane specifications of Polyvinylpyrrolidone (PVP) / polyethersulfone (PES) membranes from Spectral Diagnostics…………….14 Table 4.1 Blocking solutions used to treat the PVP/PES membranes ………………….29 Table 4.2 Blood filtration and migration on HanoRapid membranes under various blocking treatments……………………………………………………..……30 Table 4.3 Whole blood and plasma capacity of PVP/PES membranes (X,CS,C/Q-I, NX) ………………………………………………………….…………………….34 viii List of Abbreviations BSA Bovine Serum Albumin COOH Carboxyl EDC 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide·HCl HSS HEPES Sucrose Saline LFA Lateral Flow Assay MES 2-(4-Morpholino)-Ethane Sulfonic Acid NHS N-hydroxysuccinimide PBS Phosphate Buffered Saline PES Polyethersulfone PVP Polyvinyl Pyrrolidone SRB Sulforhodamine B TBS Tris Buffered Saline ix Table of Contents Abstract……………………………………………………………………………………ii Acknowledgements……………………………………………………………………….iv Biography……………………….………………………………………………..………..v List of Figures…………………………………………………………………………….vi List of Tables………………………………………………………………...…………..vii List of Abbreviations…………………………………………………………..………... ix Table of Contents………………………………………………………….…………..…..x Chapter 1 – Introduction……………………………………………………….………….1 1.1 Universal Lateral Flow Assay ..……………………………………….………1 1.2 LFA as a Diagnostic Tool for Whole Blood Sample Analysis ……..………...2 Chapter 2 – Design…………………………………………………………………….…..3 2.1 Overall Design…….……………………………………..…………….……...3 2.2 Sample Pad Selection Criteria ……………………………..…………………4 Chapter 3 – Materials and Methods…………………………………………………..…...7 3.1 Preparation of Proteins and Liposomes ……………………………………....7 3.2 Reaction Membrane Evaluation……………………………………………….8 3.21 Preparation of HanoRapid membranes for Lateral Flow Antibody Assays……………………………………………………………….9 3.22 Preparation of Millipore Hi-Flow Plus 120 Membrane Card for LFA ……………………………………………………………………...10 3.23 Hand Preparation of FUSION 5 for Lateral Flow Assays………….11 3.24 Assay Format for HanoRapid Membrane Strips……………………12 x 3.25 Assay Format for Millipore Hi-Plus 120 Membrane Strips………...12 3.26 Assay Format for FUSION 5 Strips (Immobilized with Antistreptavidin)………………………………….…………………….13 3.27 Assay Format for FUSION 5 Strips (Immobilized with Antifluorescein)………………………………………………………...13 3.3 Whole Blood Filter Membrane Evaluation ………………………………….13 3.31 Preparation of HanoRapid Membrane as Whole Blood Filters…….14 3.32 Preparation of PVP/PES Membranes as Whole Blood Filters……...15 3.33 Preparation of FUSION 5 as Whole Blood Filters…………………16 3.34 Unspecific Bindings………………………………………………...17 3.35 Whole Blood Filtration Evaluation…………………………………17 3.351 Plasma Yield ……………………………………………...17 3.352 Whole Blood Absorbance…………………………………18 3.353 Hemolysis, Erythrocyte Leakage, and Wicking Time…….18 Chapter 4 – Results and Discussion ……………………………………………………..19 4.1 Evaluation of HanoRapid as Reaction Membrane for Detection of Serum Insulin………………………………………………………………………..19 4.2 Whole Blood Filter Evaluation and Optimization…………………………...23 4.21 Nonspecific binding………………………………………………...23 4.22 Whole Blood Filtration……………………………………………..29 4.221 HanoRapid Nitrocellulose Membrane…………………….29 4.222 FUSION 5…………………………………………………32 4.223 PVP/PES Membranes……………………………………..33 xi 4.3 Optimization of Whole Blood Filtration and Signal to Noise Ratios………..39 4.31 PVP/PES membranes……………………………………………….39 4.32 FUSION 5……………………………………...…………………...45 Chapter 5 –Conclusion and Future Work………………………………………………..48 5.1 Optimization of PVP/PES plasma separation membrane……………………48 5.2 Evaluation of FUSION 5 using Latex Beads………………………………...48 References……………………………………………………….……………………….50 xii Chapter 1 – Introduction 1.1 Universal Lateral Flow Assay Lateral flow assays (LFA) are an inexpensive immunological technology that offers specific and fast result. It is particularly useful in the area of point-of care (POC) diagnostics, which eliminates the need for laboratory work conducted by trained personnel and in specialized facilities1. LFA tests can be used to detect analytes in a liquid sample, such as urine, human serum, plasma, or whole blood, which can be applied to the device in a single step. The analyte of interest could be either a low-molecularmass analyte such as drug residue, antibiotics, hormone, or a high-molecular-mass molecule such as proteins2. In general, LFAs make use of a porous membrane, usually made out of nitrocellulose that is highly adsorptive for proteins. Various types of antibodies, or ligands can then be immobilized in the capture zone of the membrane either directly or indirectly, depending on the nature of the reaction membrane. The liquid sample, is then mixed with a buffer solution and labeling agents, which could be latex beads, colloidal gold particles, dye-encapsulating liposomes, carbon black, silica, etc. The mixture is then allowed to migrate towards the capture zone by capillary forces. The analytes and/or the labeling agents are then captured by the immobilized antibodies2,3,4. There are two mechanisms by which the lateral flow assay works to produce a visible signal2. A sandwich assay, or direct assay format, is often used for the detection of high-molecular-mass analyte such as proteins. In this assay format, the analyte are sandwiched between the antibody immobilized on the test membrane and a capture 1 reagent, often another antibody, which is tagged with labeling agents to produce a signal which is indicative of the presence of the target analytes. In the other format of assay, so called the competitive format, the labeling agent is also coated with or conjugated to antibodies to the analyte of interest. But the analyte of interest, instead of the antibody, is immobilized on the membranes. As the liquid mixture reach the capture zone, analyte in the sample, then compete with the immobilized analytes for the binding sites on the antibodies tagged with the labeling agent. The absence of signal will then be an indication of a sufficient amount of analytes present in the sample5. 1.2 LFA as a Diagnostic Tool for Whole Blood Sample Analysis LFA tests are widely used for diagnostic purpose. Numerous test strips have been developed for testing of drugs of abuse, infectious diseases, pregnancy, etc 3,6,7. Since it is often developed as a POC device, sample preparation must be completed prior to the test analysis on the test strip9. This then becomes difficult when it comes to heterogeneous liquid samples such human whole blood, which are traditionally isolated into serum or plasma in laboratories because of its high cellular fractions8,10. Although there are many lateral flow assays commercially available for diagnostic purposes, few could directly work with human whole blood samples11. While there are many commercially available whole blood filters, scarce information is available on how to select and incorporate these materials into a lateral flow assay. Therefore, there exists a need for the development of a universal method that can efficiently and consistently separate whole blood for lateral flow assays. 2 Chapter 2- Design 2.1 Overall Design A typical lateral flow strip test is composed of a reaction membrane, usually made out of nitrocellulose, a sample pad, a conjugate pad, and an absorbent pad. The conjugate pad, commonly made out of glass fiber filter, paper filters, and surface-treated (hydrophilic) propylene filters, is used to store and deliver the detector agent, which are dye encapsulating liposomes in this case. Please note that a conjugate pad will not be used in this project design, since lateral flow assays will be run immediately for the experimental purpose. The absorbent pad, which is placed at the end of the test strip, serves as the sink for the sample as it migrates through the strip. The absorbent pad is often made out of cellulosic paper. It works to enhance the assay sensitivity by increasing the total amount of volume of sample that can be accommodated on the membrane. Lastly, the sample pad, which will be placed at the beginning of the test strip, acts as a filtration device by removing undesirable fractions of the liquid sample. It also serves to absorb the sample and provide a uniform flow on the test strip. For high viscosity and high concentration of particles in fluid, such as whole blood samples, special blood separation filters should be used2,3. The focus of the project will be on sample pad selection and optimization. A schematic of the device is shown in Figure 2.1. 3 Sample Pad Nitrocellulose Membrane Plastic backing sheet Absorbent pad Test Control line line Flow direction Figure 2.1 Schematic of the lateral flow assay used in this study. The top schematic is the side view of a test strip. The bottom schematic is the top view of a test strip. Figure is not drawn to scale. The main objective is the evaluation of the performance of the filtering device for the detection of an analyte that is normally detectable in whole blood. Thus, in preparation for this evaluation, a basic lateral flow assay will be developed with a detection of limit low enough for the serum concentration of the analyte. Alternatively, whole blood will be spiked with the analyte which will then be used as a replacement for the evaluation of whole blood filters for the device. 2.2 Sample Pad Selection Criteria Selection of a whole blood filter membrane will largely be based on a manufacture’s claims on membrane efficiency and membrane specifications. The suitability of a certain membrane type as a sample pad is determined by plasma yield, 4 absorbance capacity, degree of hemolysis, leakage of red blood cells, and wicking speed9,10,11. The sensitivity of the assay is largely dependent on the volume of the plasma separated out of the whole blood sample, which is partially representative of the quantity of the analyte collected. The plasma yield is determined by first, the absorbance capacity of whole blood, and secondly by the membrane’s ability to extract the maximum volume possible out of absorbed blood. Generally, filter materials that provides high plasma yield will perform better for an assay since larger sample volumes can be analyzed. Other factors to be considered include low degree of hemolysis, a process during which the erythrocyte’s cell membrane ruptures. Hemolysis is highly undesirable in medical tests because it will cause turbidity of extracted plasma flow and increased background noise. Similarly, erythrocyte leakage could also contaminate the background of the test membrane and significantly reduce the sensitivity of the test. Ideally, erythrocyte leakage can be reduced by restricted pore size and other manufacturing limitations10. Shortening wicking time is also crucial to the efficiency of lateral flow assay test. Wicking time of the membrane can be greatly affected by the saturation of the membrane via addition of buffer. The deposit of blood cells at various parts of the sample pad could also increase wicking time by several orders of magnitudes, rendering the test inefficient. Therefore, we evaluated here various membranes for their blood filtering performances based on the above criteria. For the analyte of interest to be captured and detected, an efficient whole blood filtration must first take place. Subsequently, the filter 5 aimed to minimize unspecific bindings and produce higher signal/noise ratio was optimized. 6 Chapter 3 – Materials and Methods 3.1 Preparation of Proteins and Liposomes Insulin from Sigma-Aldrich(st Louis, MO) was diluted in 1x Phosphate Buffered Saline (PBS) buffer (pH 7.4) to the final concentrations of 0.05, 0.5, 0.75, 1.0, 1.25, 2.5, 5.0, 1, 5, 10, 100 µg mL-1. Anti-insulin #E86306M and #E86802M (Biodesign, ME, USA) were diluted in 1xPBS buffer to the final concentration of 500 µg mL-1. Anti-streptavidin (Vector Labs #SP-4000) was diluted to 1 mg/mL in 0.4 M NaHCO3/Na2CO3, pH 9.0. Biotinylated anti-fluorescein (Vector Labs #BA0601) was diluted to 40 µg mL-1 in 1x PBS. The liposomes used in this study were prepared by Dr. Katie Edwards, Barb Leonard, and Kit Meyers using the reverse phase evaporation method. Protein was conjugated to COOH-tagged sulforhodamine B encapsulating liposomes using N-hydroxysuccinimide (NHS) (NovaBiochem, #A27868) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Pierce, #22980, Lot#HK108905), based on the protocol14 developed by Dr. Katie Edwards. First, the amount of total lipid (TL) in nmol present in the desired volume of liposomes was calculated by multiplying the desired volume of liposomes by the phospholipid concentration (PL) of the free liposomes. The amount of protein needed for conjugation was then determined by multiplying the TL amount obtained earlier by the TL mol%, typically 0.05 mol %. The amounts of EDC and NHS needed for conjugation were determined based on the rule that 0.3 nM equivalents EDC and NHS was used for each nmol of the TL content. The EDC and NHS, both were then diluted to 100 mg/mL with 0.1M MES buffer, pH 6.0. The desired volume of COOH- 7 tagged liposomes was then added to a microcentrifuge tube. The EDC and NHS were subsequently added to the side of the centrifuge tube containing the liposomes. The tube was immediately vortexed for about 30 seconds and stored in the dark for 15 minutes. The protein was then added to the side of the microcentrifuge and vortexed for 30 seconds. The mixture was then incubated in the dark for 2 hours with periodic mixing. The conjugated liposomes where subsequently purified through a SEC column packed with Sepharose CL-4B using 1xHSS as an elution buffer. The most concentrated fractions were collected to be used for lateral flow assays. The Bartlett assays were subsequently completed by Barb Leonard or Kit Meyers to determine the PL of the conjugates. All conjugated liposomes were diluted in 1xHSS to a final PL of 1 mM before used. 3.2 Reaction Membrane Evaluation HanoRapid nitrocellulose membrane (Hamony, Cheshire,CT), Millipore HiFlow Plus 120 membrane cards (Millipore, Billerica, MA), and FUSION 5 (Whatman, FlorhamPark, NJ ) were examined for their binding specificity. The Table 3.1 lists the membrane specifications provided by manufacturers. 8 Table 3.1. Important membrane specifications of HanoRapid Nitrocellulose membrane, FUSION 5, Millipore Hi-Flow Plus 120 Membrane Cards. Materials Pore Size Protein Wicking Time Back Support Binding HanoRapid (Hanomy) Fusion 5 (Whatman) Millipore HiFlow Plus 120 Membrane Cards Nitrocellulose 5 µm High - PET film Not disclosed. 11 µm None/low N/A Nitrocellulose - High 2min 40secs/ 7.5cm 120 ± 30 sec / 4cm Polystyrene, white 3.21 Preparation of HanoRapid membranes for Lateral Flow Antibody Assays The following reagents were used for the preparation of HanoRapid nitrocellulose membranes for LFA: 10xTris-buffered saline (0.2 M Trizma base, 1.5 M sodium chloride, 0.1%(w/v) sodium azide, adjusted to pH 7.0 with 25% (w/v) HCl; 10x PBS; 2% Polyvinylpyrrolidone (PVP); 0.15% casein; 1% gelatin; 10% BSA, 5% Tween 20, Methanol. The original protocol15 for the preparation of HanoRapid nitrocellulose membranes was provided by Dr.Katie Edwards. Raw HanoRapid membrane was cut into a sheet of size of 20 cmx7.5 cm. The membrane sheet was then pre-wet by soaking it in 100 mL of pre-wetting solution containing 1xPBS and 10% methanol. The excess solution was then blot off with Kimwipes. The membrane sheet was subsequently dried in the vacuum oven at 40°C and under 15" Hg for 30 minutes. On the sheet, 500 µg mL-1 anti-insulin #E86306M was coated in a test line (2.5 cm from the proximal end) using a Linomat IV TLC Applicator (Camag Scientific, Wrightsville Beach, NC) at 4 µL/Sec. The total volume of antibody applied was 38 µL per sheet. The membrane sheet was 9 subsequently dried in vacuum oven at 40°C and under 15" Hg for 1.5 hours. The membrane was then soaked in a blocking solution listed in Table 3.2 with the laminated side down in a Tupperware container. The container was then placed on a shaker incubator at room temperature using a slow agitation for 30 minutes. The excess blocking solution was blot off with Kimwipes, and the membrane sheet was dried at 25-30°C and under 15" Hg for 2.5 hours. It was then removed from the oven and cut into strips (4.5 mm x 7cm). They were stored at 4°C in a vacuum sealed bag until used. Table 3.2. Blocking solutions used with HanoRapid membranes. The effects of blocking solutions 1-6 were compared in the first part of HanoRapid evaluation. The effects of blocking solution 7-11 were compared in the second part of HanoRapid evaluation, where blocking solution was optimized for higher test sensitivity. 1 2 3 4 5 6 7 8 9 10 Blocking Solutions 0.10% gelatin, 0.02% PVP, 0.05% Casein, 1xTBS, 0.002% Tween20 0.05% gelatin, 0.02% PVP, 0.005% casein, 1xTBS, 0.002% Tween20 0.10% gelatin, 0.02% PVP, 0.00% casein, 1xTBS, 0.002% Tween20 0.10% BSA, 0.02% PVP, 0.005% Casein, 1xTBS, 0.002% Tween20 0.05% BSA, 0.02% PVP, 0.005% Casein, 1xTBS, 0.002% Tween20 0.10% BSA, 0.02% PVP, 1xTBS, 0.002% Tween 20 0.02%PVP, 0.005%Casein, 1XTBS, 0.002% Tween20 0.01% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20 0.02% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20 0.03% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20 3.22 Preparation of Millipore Hi-Flow Plus 120 Membrane Card for LFA The following reagents were used for the preparation of Millipore Hi-Flow Plus 120 Membrane Card for LFAs: 10x Phosphate Buffered Saline (PBS); 2% Polyvinylpyrrolidone(PVP); 1% sodium casein; 10% BSA, 2.0 M sucrose, 5% Tween 20. 10 The protocol for the preparation of Millipore Hi-Flow Plus 120 was modified based on the protocol for the HanoRapid membrane preparation15. The following changes were made. First, the membrane card was cut into the size of 6 cm x 20 cm. Second, the pre-wetting step was removed. Third, the antibody was applied differently. Anti-insulin #E86802M (500 µg mL-1) was immobilized in a test line (3.3 cm from the proximal end of the membrane card), and 1 mg mL-1 anti-streptavidin was immobilized in a control line (3.8 cm from the proximal end). Forth, the blocking solution was changed to a buffer solution containing 1xPBS, 0.25% sucrose, 0.002% Tween 20, 0.1% sodium casein. Lastly, prior to cutting, a 4cm x 20 cm absorbent pad (Millipore, Billerica, MA) was adhered to the distal end of the membrane card with no more than 2 mm of overlap with the nitrocellulose portion. 3.23 Hand Preparation of FUSION 5 for Lateral Flow Assays The following reagents were used for the preparation of FUSION 5 for LFAs: 10xTris-buffered saline (0.2 M Trizma base, 1.5 M sodium chloride, 0.1%(w/v) sodium azide, adjusted to pH 7.0 with 25% (w/v) HCl); 10x Phosphate Buffered Saline (PBS); 2% Polyvinylpyrrolidone(PVP); 0.15% casein; 10% BSA, 2.0 M sucrose, 5% Tween 20. The protocol for the preparation of FUSION 5 was modified based on the protocol15,18 listed in 3.21. The following changes were made. First, the membrane was adhered to a backing card (Millenia Diagnostics, San Diego, CA) before any treatments. It was then cut into strips by the size of 4.5 mm x 7 cm. Second, the pre-wetting step was removed. Third, the antibody was applied differently. Here, 1µLof 1 mg/mL antistreptavidin or biotin conjugated 40µg/mL anti-fluorescein was applied with a pipette 11 1.5cm up from the base of the strip. Lastly, half of the strips prepared were unblocked, while the rest were treated with a blocking solution containing 0.005% casein, 0.02% PVP, 0.002% Tween 20, 1xTBS, 1% gelatin. 3.24 Assay Format for HanoRapid Membrane Strips An insulin solution (5µL) or 1xPBS (5µL) was placed in a glass test tube (10 x 75 mm). A test strip was then inserted into the test tube. After the solution was absorbed (about 1min), 5µL of liposomes conjugated to anti-insulin was added to the same glass tube and allowed to be absorbed, followed by the addition of 30 µL of 1xHSS. Once the 1xHSS was completely absorbed onto the strip (about 5 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively17. 3.25 Assay Format for Millipore Hi-Plus 120 Membrane Strips Prior to tests, the bottom of the membrane card (2 cm long portion below the nitrocellulose membrane) was removed because sample pad was not needed for this part of the experiment. An insulin solution (5 µL) or 1xPBS (5 µL) was placed in a glass test tube (10 x 75 mm), and a test strip was subsequently inserted into the test tube. After the solution was absorbed (after about 1 min), 5 µL of liposomes conjugated to anti-insulin was added to the same glass tube and allowed to be absorbed, followed by 5 µL of streptavidin-tagged liposomes, then followed by 50 µL of 1xHSS. Once the 1xHSS was completely absorbed onto the strip (about 5 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively. 12 3.26 Assay Format for FUSION 5 Strips (Immobilized with Anti-streptavidin) The streptavidin-tagged liposomes (5 µL) was added to a glass tube (10 x75 mm), and a test strip with immobilized anti-streptavidin was subsequently inserted into the test tube. The liposomes were allowed to be fully absorbed, followed by 50 µL of 1xHSS. Once the 1xHSS was completely absorbed into the strip (about 5 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively. 3.27 Assay Format for FUSION 5 Strips (Immobilized with Anti-fluorescein) The fluorescein -tagged liposomes (5 µL) was added to a glass tube (10 x75 mm), and a test strip with anti-fluorescein immobilized was subsequently inserted into the test tube and allowed to be absorbed, followed by 50 µL of 1xHSS. Once the 1xHSS was completely absorbed into the strip (about 5 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively. 3.3 Whole Blood Filter Membrane Evaluation The following membranes were evaluated for their whole blood filtering performances. Polyvinylpyrrolidone (PVP)/polyethersulfone(PES) Primecare membranes (Spectral Diagnostics, Toronto, Canada), FUSION 5 (Whatman, FlorhamPark, NJ ), and HanoRapid nitrocellulose membrane (Hanomy, Cheshire, CT). Two categories of PVP/PES membranes, as provided by the manufacturer were examined. The separator membranes performed blood cell/plasma separation. The other category, the collector membranes, performed plasma collection. There were two types of separator membranes, 13 X and S/G and three types of collector membranes, which were C/S, C/Q, and NX, respectively. Table 3.3 lists the PVP/PES membranes’ specification provided by the manufacturer. Table 3.3. Important membrane specifications of Polyvinylpyrrolidone (PVP)/polyethersulfone(PES) membranes from Spectral Diagnostics (Ontario, Toronto). Type of Membrane Separator Code Average Thickness S/G 260-300 µm with CV of 5% Collector C/S 200-300 µm with CV of 5% Porosity Gradient ~ 35 µm (top) ~ 2.5µm(bottom) ~ 35 µm (top) ~2.5µm(bottom) Collector C/Q 130-200 µm with CV of 3% ~ 12 µm (top) ~ 3µm(bottom) Separator X ~ 35 µm (top) ~5 µm(bottom) Separator NX 160 -200 µm with CV of 5% 210-250 µm with CV of 5% ~ 35 µm (top) ~2.5 µm(bottom) Capacity 50 µL of whole blood 15-22 µL/cm2 depending on membrane thickness 12-16 µL per cm2 Depending on membrane thickness 40 µL of plasma from 110 – 120 µL of whole blood per cm2 35 µL of plasma from 100 of whole blood per cm2. Depending upon hematocrit 3.31 Preparation of HanoRapid Membrane as Whole Blood Filters HanoRapid membranes used for this part of the study were prepared with the protocol listed in 3.21. 14 3.32 Preparation of PVP/PES Membranes as Whole Blood Filters PVP/PES membranes were adhered, as sample pads, onto Millipore Hi-Flow Plus 120 Membrane Cards prepared with the protocol listed in 3.22. A typical set up for the incorporation of PVP/PES membranes into Millipore Hi-Flow Plus 120 Membrane Card is illustrated in Figure 3.1. The collector membrane was adhered just below the nitrocellulose portion of the membrane card, with an overlap no more than 2 mm. The separator (blue) was adhered to the membrane card below collector, with an overlap no more than 2 mm. The absorbent pad (4 cm x 20 cm) was adhered to the distal end of the membrane card, with an overlap to the membrane less than 2 mm. The size of the collector membrane and separator membrane will vary in the following sections for the experiments designed to evaluate different aspect of the membranes’ blood filtering performances. The sizes will be specified in the following sections respectively. 15 Sample Pad Separator Collector Plastic backing sheet Nitrocellulose Membrane Absorbent pad Test Control line line Flow direction Figure 3.1 A typical Millipore Hi-Flow Plus 120 membrane card with PVP/PES membranes provided by Spectral Diagnostics. The collector (red) is first adhered to the membrane card just below the membrane portion, with an overlap no more than 2mm. The separator (orange) is adhered to the membrane card below collector, with an overlap no more than 2 mm. The absorbent pad (grey) is adhered to the distal end of the membrane card. The overlap between the nitrocellulose membrane and the absorbent pad should be no more than 2 mm. Figure is not drawn to scale. 3.33 Preparation of FUSION 5 as Whole Blood Filters FUSION 5 membranes were adhered, as the sample pad, onto Millipore Hi-Flow Plus 120 Membrane Cards prepared with protocol listed in 3.22. Similarly to the set up shown in Figure 3.1, FUSION 5 (4 cm x 20 cm) was adhered below the nitrocellulose portion of the membrane card (6 cm x 20 cm). There should be an overlap (<2 mm) between FUSION 5 and the nitrocellulose membrane. 16 3.34 Unspecific Bindings Unspecific binding on the blood filtering materials reduces the amount of liposomes and analytes that are able to reach the reaction membrane. Therefore, the amount of unspecific binding on the sample pad could be estimated by comparing the signals/noise ratio of the test strips with and without sample pads. 3.35 Whole Blood Filtration Evaluation Fresh healthy human whole blood was collected and obtained at Gannet Cornell lab in K2-EDTA Vacutainer tubes. Whole blood samples were directly pipetted out of the tube after thorough mixing. To obtain plasma, 2 mL of fresh whole blood was first diluted with 2 mL of balanced salt solution, and was then carefully layered on top of 3 mL of Ficol. The sample was then centrifuged at 19°C for 30 minutes at 400 x g. The plasma layer was subsequently removed and stored in a new tube at 4°C. Drawn blood and isolated plasma older than 24 h were discarded. 3.351 Plasma Yield First, to approximate the volume of plasma separated by migration distance observed on the collector, standards were created using known volumes of plasma. PVP/PES membranes were prepared using the protocol listed in 3.5 with one modification. Only the collector membrane (4 cm x 20 cm) was adhered to the membrane card (6 cm x 20 cm) below the nitrocellulose membrane, with an overlap (<2 mm) between the two materials. To determine the plasma yield on PVP/PES collector membranes, plasma was dyed with 1µg of sulforhodamine B (SRB), yielding a bright 17 purple colored sample. A known volume of dyed plasma (6 µL, 8 µL, and 10 µL) was added to a test tube (10 x 75mm). A test strip was then inserted and allowed to completely absorb the plasma. The distance of the migration was subsequently measured and used as a standard value for each corresponding volume. The plasma yield could then be roughly quantified by comparisons against the standards. 3.352 Whole Blood Absorbance To determine the absorbance capacity of a selected membrane, a known volume of whole blood (ranged from 20 to 100 µL) was first pipetted into a 12 mm x 75 mm test tube. A test strip, either a HanoRapid strip (prepared in 3.4), a raw FUSION 5 strip (4.5mm x 7cm), or a test strips with PVP/PES as sample pad (prepared in 3.5) was placed into the test tube and allowed to be saturated with the blood. Any unabsorbed blood volume was measured to calculate the true absorbed volume. 3.353 Hemolysis, Erythrocyte Leakage, and Wicking Time Hemolysis, displayed as a reddish cloudiness, can be directly observed on the membrane. Similarly, the extent of erythrocyte leakage could also be estimated by simply observation. The most likely region for erythrocyte leakage is the border between the sample pad and reaction membrane. Wicking speed of each sample pad was determined by measuring the time necessary to first complete whole blood filtration, and then to complete one full test . Since most commercial strip tests have an about 10 minutes assay time, it is necessary to reduce the test time for our assay to a reasonable range. 18 Chapter 4 – Results and Discussion 4.1 Evaluation of HanoRapid as Reaction Membrane for Detection of Serum Insulin Based on an earlier protocol for antibody-based lateral flow assays, an LFA for insulin was developed and optimized with respect to its limit of detection. Initially, the blocking solution was optimized in order to minimize any unspecific binding. Initial screening showed that two blocking solutions containing 0.10% gelatin, 0.02% PVP, 0.005% Casein, 1x TBS, 0.002%Tween 20 and 0.05% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20, gave relatively low background noises and strong signals for the detection of insulin at 10 µg/mL & 100 µg/mL (Figure 4.1). When the insulin concentration was varied between 0 and 10 µg/ml, insulin was detected as low as 1 µg/mL on the membrane blocked with 0.05% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20. However, the BSA blocked membranes did seem to induce more background noises than the gelatin blocked membranes as previously noted (Figure 4.2). 19 Figure 4.1 Lateral flow assays for detection of 1xPBS buffered insulin on HanoRapid. Insulin were at 0,10,100 μg mL-1 with anti-insulin #E86306M at 0.5 µg/mL-1 immobilized to HanoRapid strips 2.5 cm from the base. Liposomes conjugated to antiinsulin #E86802M (5 μL) were applied to each strip of membrane, followed by the addition of 30 μL 1xHSS. Membranes were treated with different blocking solution. From top to bottom: 1) 0.10% gelatin, 0.02% PVP, 0.05% Casein, 1xTBS, 0.002% Tween20; 2) 0.05% gelatin, 0.02% PVP, 0.005% casein, 1xTBS, 0.002% Tween20; 3) 0.10% gelatin, 0.02% PVP, 0.00% casein, 1xTBS, 0.002% Tween20; 4) 0.10% BSA, 0.02% PVP, 0.005% Casein, 1xTBS, 0.002% Tween20; 5) 0.05% BSA, 0.02% PVP, 0.005% Casein, 1xTBS, 0.002% Tween20; 6) 0.10% BSA, 0.02% PVP, 1xTBS, 0.002% Tween20. Blocking 1) and 5) showed most promising results in terms of the high signal to noise ratio displayed. The negative control blocked with blocking 2) should be ignored because the strip was stuck to the side of the test tube during the experiment. 20 Figure 4.2. Lateral flow assays for the detection of 1xPBS buffered insulin at 0, 0.05, 0.5, 0.75, 1.0, 1.25, 2.5, 5.0, 7.5, and 10 µg/mL using HanoRapid nitrocellulose membranes with anti-insulin #E86306M at 0.5 µg/mL-1 immobilized to HanoRapid strips 2.5 cm from the base.. Liposomes conjugated to anti-insulin #E86802M (5 μL) were applied to each strip of membrane, followed by the addition of 30 μL 1xHSS. Left: membranes were blocked with 0.05% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20. Right: membranes were blocked with 0.10% gelatin, 0.02% PVP, 0.005% Casein, 1XTBS, 0.002%Tween 20. Note, due to a limitation in the image resolution of the scanned image, the positive signal on membrane blocked with BSA at 1µg/mL was not visible here. However, the clinical serum insulin conc. range is 36-179 pM, which was substantially lowered than 1µg/ml17, 18. We had previously noticed that lowering the BSA concentration in the blocking solution seemed to enhance both the signal and noise of the test. In an attempt to increase the sensitivity of the test, the concentration of BSA was varied from 0-0.03% in another batch of blocking solutions while keeping other chemicals at the same conc. Results were shown in the Figure 4.3. Lowering BSA concentration overall increased noises in the background. Membranes blocked with 0.03% BSA detected insulin as low as 0.75 µg/mL. Nonetheless, there was still at least a two orders of magnitude difference between current sensitivity and the serum insulin concentration. Therefore, it led us to conclude that HanoRapid membranes using a 21 traditional lateral flow assay are not sensitive enough for the detection of serum insulin. Thus, for the purpose of the study of blood filter membranes, whole blood spiked with insulin was used from this point on. Figure 4.3. Lateral flow assays for the detection of 1xPBS buffered insulin at 0, 0.5, 0.75, 1.0, 1.25, 2.5, 5.0, 7.5, and 10 µg/mL using HanoRapid nitrocellulose membranes. Liposomes conjugated to anti-insulin #E86802M (5 μL) were applied to each strip of membrane, followed by the addition of 30 μL 1xHSS. Top left: membranes were blocked with0.02%PVP, 0.005%Casein, 1XTBS, 0.002% Tween20. Top right: membranes were blocked with0.01% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20. Bottom left: membranes were blocked with 0.02% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20. Bottom right: 0.03% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002%Tween20. A positive signal was detected at 0.75 µg/mL when the membranes were treated with the blocking solution containing 0.03%. Note, membranes blocked with 0.03% BSA, 0.02%PVP, 0.005%Casein, 1XTBS, 0.002% Tween20 were not tested at concentration. above 0.75 µg/mL due to an insufficient supply of conjugated liposomes. 22 4.2 Whole Blood Filter Evaluation and Optimization 4.21 Nonspecific binding A good whole blood filter must exhibit minimum nonspecific bindings, so that it will not interfere with the reaction dynamics of the assay. HanoRapid nitrocellose membrane already demonstrated little non-specific binding with the appropriate blocking treatment as previously shown (Figure 4.2). This part of the results and discussion will focus on FUSION 5 and PVP/PES membranes. FUSION 5 is non-protein binding in nature, therefore antibodies cannot be directly immobilized on the membrane surface as in the traditional method. Based on this, it would be expected signals to be weak or even invisible if antibodies were immobilized onto the membrane membranes directly. To test this, membranes were hand-spotted with biotinylated anti-streptavidin and anti-fluorescein 2.5 cm from the bottom of the membranes, respectively. As anticipated, even though signals were visible, its intensity was very low probably due to insufficient antibody adsorption to the membrane material. Unblocked membranes were compared to those blocked with 0.005% Casein, 0.02% PVP, 0.002% Tween-20, 1xTBS, 1% gelatin to examine if blocking would have any effects on surface binding properties. Results showed that blocking increased the noises in the background, i.e. non-specific binding of liposomes (Figure 4.4). 23 Figure 4.4. Lateral flow assays on FUSION 5 membranes strips with hand-spotted biotinylated 1 mg/mL anti-streptavidin (LEFT) and 40 µg /mL anti-fluorescein(RIGHT). Top membrane strips were unblocked and the bottom strips were blocked with 0.005% Casein, 0.02% PVP, 0.002% Tween-20, 1xTBS, 1% gelatin. Streptavidin-tagged or fluorescein-tagged liposomes ( 5µL) was added to a glass tube(10 x75 mm), and a test strip with the corresponding antibody immobilized was subsequently inserted into the test tube and allowed to be absorbed, followed by 50 µL of 1xHSS. Positive signals were obtained on both unblocked strips. Next, non-specific bindings associated with polyvinylpyrrolidone (PVP)/polyethersulfone(PES) membranes to be used as sample pads were examined. There PVP/PES membranes provided by Spectral Diagnostics are categorized into two groups, based on their functionality. The separator membranes, X and S/G, perform blood cell/plasma separation. The other category, the collector membranes, which consists of C/S, C/Q, and NX, perform plasma collection. These membranes are different in their thickness and pore sizes. Table 3.3 lists the PVP/PES membranes’ specification provided by the manufacturer. These PVP/PES membranes were made of the same materials, and they should exhibit similar binding affinities. Therefore, X and CS were randomly selected from each category for the first part of the experiment. They were added to Millipore Hi-Flow Plus 120 membrane cards as the sample pad. Figure 4.5 showed the lateral flow assay results performed with these two materials. Strip #1 and strip #2 were the negative and positive controls, respectively. Strip #3, on which 5 µL of 5 µg/mL PBS buffered insulin solution, 5 µL anti-insulin #E86306M tagged liposomes and 5 µL streptavidin tagged liposomes, and 50µL 1xHSS were applied consecutively, showed no 24 signals. It was suspected that the insulin might have been withheld at the sample pad, so that there wasn’t enough bound at the test line. Therefore, the volume of the 5 µg/mL insulin solution applied was increased to 7 µL and 10 µL on the next two strips tested, respectively. Signal then became slightly detectable at 10µL, indicating a possible holdup of insulin at the sample pad. However, it was also possible that there might have a liposome hold-up as well because it was observed that the collector portion of the sample pad overall was slightly pinkish, indicative of trapped liposomes. To determine the major cause for the weak signals, instead of applying liposomes at the base of the strip and allowing them to migrate through the sample pad, they were hand-spotted above the sample pad region in strip #6. The absence of signal indicated that the loss of liposomes noted before at the collector membrane was not the major reason for signal reduction. Finally, strip #7, which had sample pad completely removed, showed signals that were comparable to the signal on strip #2, indicating that the reduction of signal was due to unspecific binding of insulin at sample pad comprised of the PVP/PES membranes, X and CS. 25 Figure 4.5. Lateral flow assay on Millipore Hi-Flow plus 120 membrane cards with PVP/PES membranes (X/CS) as the sample pads. Millipore Hi-Flow 120 had anti-insulin #E86802M at 0.5 µg/mL-1 immobolized as a test line and anti-Streptavidin at the control line. Strip #1 and #2 were the negative and positive control, respectively. On strip #3, #4, and #5, 5, 7, 10 µL of 5µg/mL PBS buffered insulin solution were applied respectively, followed by 5µL anti-insulin #E86306M tagged liposomes and 5µL streptavidin tagged liposomes, and 50µL 1xHSS. Strip # 6, 7 were the same as #3 except that liposomes were applied above the sample pad on strip #6, and strip #7 had sample pad completely removed before LFA. Previously, Kim and Choi had noted that treating the sample pad with 50 mM borate buffer, pH 7.4, containing 1% BSA, 0.05% Tween-20, 0.05% sodium azide, minimized the effects of sample pad on the migration of gold-antibody conjugate21. The same approach was adopted here to reduce insulin non-specific binding. Blocking solutions of varying BSA, Tween-20, casein concentration were made and used to treat the PVP/PES membranes. Because all membranes should have similar surface binding properties, a preliminary screening experiment for the most effective blocking solution was performed with membrane X as the sample pad. A total of 7 blocking solutions with varied BSA, sodium Casein, and Tween-20 concentrations were compared (Table 4.1). Results showed obvious improvement of signal on all blocked membranes in Figure 4.6. It was observed immediately after the tests that membranes blocked with solutions #3, #5, and #6 had the strongest signals among the membranes. Thus, 1% of BSA and 0.1% of 26 sodium casein was the appropriate concentration in the blocking solution. Figure 4.7 showed that the blocking solutions were equivalently effective on membrane NX, C/Q-I, and CS. Figure 4.6 Lateral flow assay results on Millipore Hi-Flow Plus 120 using the PVP/PES blood filtering membrane X from Spectral Diagnostics. X was treated with one of the 7 different blocking solutions in Table 4.1, respectively. Millipore Hi-Flow 120 had antiinsulin #E86802M at 0.5 µg/mL-1 immobilized as a test line and anti-Streptavidin at the control line. PBS buffered insulin solution at 5 µg/mL (10 µL) was detected on all the strips. 27 Figure 4.7 Lateral flow assay results on Millipore Hi-Flow Plus 120 using the PVP/PES blood filtering membrane NX, C/Q, CS from Spectral Diagnostics. The filtering membrane was treated with the blocking solutions #3, #5, #6 in Table 4.1, respectively. Millipore Hi-Flow 120 had anti-insulin #E86802M at 0.5 µg/mL-1 immobilized as a test line and anti-Streptavidin at the control line. PBS buffered insulin solution at 5µg/mL (5µL) was detected on all the strips. Signals were absent on the unblocked test strips with NX and CS and weakly detectable on C/Q-I. Signals were equivalently strong based on visual inspection on all blockedtest strips. 28 Table 4.1. Blocking solutions used to treat the PVP/PES membranes. 1 2 3 4 5 6 7 Blocking Solutions 0.03% BSA, 0.02% PVP, 0.005% Sodium Casein, 1x TBS, 0.002% Tween20, 0.05% sodium azide 0.1% BSA, 0.02% PVP, 0.01% Sodium Casein, 1x TBS, 0.002% Tween20, 0.05% sodium azide 1% BSA, 0.02% PVP, 0.005% Sodium Casein, 1x TBS, 0.002% Tween20, 0.05% sodium azide 0.1% BSA, 0.02% PVP, 0.01% Sodium Casein, 1x TBS, 0.01% Tween20, 0.05% sodium azide 0.1% BSA, 0.02% PVP, 0.1% Sodium Casein, 1x TBS, 0.002% Tween20, 0.05% sodium azide 1% BSA, 0.02% PVP, 0.1% Sodium Casein, 1x TBS, 0.002% Tween20, 0.05% sodium azide 0.1% BSA, 0.02% PVP, 0.5% Sodium Casein, 1x TBS, 0.5% Tween20, 0.05% sodium azide 4.22 Whole Blood Filtration 4.221 HanoRapid Nitrocellulose Membranes Among the three selected membrane types, HanoRapid had the smallest blood absorbance capacity. Initial screening showed that the maximal blood volume absorbed was approximately 20 µL within 40 minutes overall. Experiments also showed that blocking solutions only slightly affected the membrane’s performance in whole blood filtration. Figure 4.8 showed that the membrane blocked with blocking solution #1 containing 0.10% gelatin, 0.02% PVP, 0.006% Casein, 1xTBS, 0.002% Tween-20 and another blocking solution #5 containing 0.05% BSA, 0.02% PVP, 0.005% Casein, 1xTBS, 0.002% tween-20 had somewhat greater capacity for whole blood than the other membranes. All the membranes except for membrane blocked with the solution (containing 0.10% gelatin, 0.02% PVP, 0.006% Casein, 1xTBS, 0.002% Tween-20) 29 showed a considerable amount of hemolysis. HanoRapid membrane was able to retain red blood cells, and allow mostly plasma to flow up. However, when 25 µL of whole blood was spiked with PBS-buffered insulin (5 µg/mL) was filtered and analyzed with membranes blocked with solution #1 and# 5 (see Table 4-1), neither membrane showed a positive signal (Figure 4.9). In addition, hemolysis became more apparent with the addition of HSS buffer with visible liposomes hold up at the bottom of the membrane. These observations led to the conclusion that the HanoRapid membrane was not a suitable whole blood filter. Table 4.2. Blood filtration and migration on HanoRapid membranes under various blocking treatments. Different blocking solutions seemed to have different effects on how blood would migrate on HanoRapid membranes, in terms of distance as well as the rate of migration. Note that red blood cells and other cell fractions were retained at 0.7 cm – 2.3 cm from the base of the membrane depending on the blocking. Blood cell fractions migrated up the membrane more than 2 cm on the strips with blocking #A and #E. The volume of blood migrated was estimated to be less than half of the blood sample volume applied (50 µl), implicating a possible range of capacity of the membrane for whole blood. Blocking 1 Blocking 2 Blocking 3 Blocking 4 Blocking 5 Distance Red blood cells and other cell fractions migrated (cm) Distance serum migrated (cm) Estimated time for complete migration (min) Relative Rate of blood migration (0.10% gelatin, 0.02% PVP, 0.05% Casein, 1XTBS, 0.002% Tween 20) (0.05% gelatin, 0.02% PVP, 0.005% casein, 1X TBS, 0.002% Tween 20) (0.05% gelatin, 0.02% PVP, 0.00% casein, 1X TBS, 0.002% Tween 20) ( 0.10% BSA, 0.02% PVP, 0.005% Casein, 1X TBS, 0.002% Tween 20) ( 0.05% BSA, 0.02% PVP, 0.005% Casein, 1X TBS, 0.002% Tween 20) 2.3 1.9 1.5 1.7 2.1 5.9 4.8 4.2 5.2 5.8 25 30 35 35 Not recorded fast fast slow medium Not recorded 30 Figure 4.8. Blood filtration/migration on Hanorapid membranes. Each membrane strip was treated with a different blocking solution, as indicated by the number next to the strip (Table 4-2). 30 µL of whole blood was applied in a test tube, and the strip (4.5mm x 7mm) was inserted into the test tube and allowed to absorb as much blood as possible in 40 min. Strips that were blocked with 0.10% gelatin, 0.02% PVP, 0.05% Casein, 1XTBS, 0.002% Tween 20, and 0.05% BSA, 0.02% PVP, 0.005% Casein, 1X TBS, 0.002% Tween 20 respectively absorbed approximately 20 µL of whole blood. The other strips absorbed less as shown by the shorter migration distance. Figure 4.9 Filtration of whole blood (25 µL) spiked with PBS-buffered insulin (5 µg/mL). Sample was filtered and analyzed with HanoRapid membranes strip (top) blocked with solution( 0.10% gelatin, 0.02% PVP, 0.05% Casein, 1XTBS, 0.002% Tween 20) and another (bottom) blocked with solution (0.05% BSA, 0.02% PVP, 0.005% Casein, 1X TBS, 0.002% Tween 20). Anti-insulin #E86306M at 0.5 µg/mL-1 was immobilized to HanoRapid strips 2.5 cm from the base. Liposomes conjugated to antiinsulin #E86802M (5 μL) were applied to each strip of membrane after whole blood, followed by the addition of 30 μL 1xHSS. 31 4.222 FUSION 5 Based on the test data provided by Whatman, FUSION 5 has a maximum pore size of 11 µm and a thickness of 370 µm, which explained the greater absorbance capacity. In the experiment where different volumes of whole blood were allowed to migrate up the membrane strips (4.5mm x 6cm), it was observed that 30 µL of whole blood was fully absorbed by the membrane within 2 minutes and 50 µL was absorbed in no more than 4 minutes on another membrane. As shown in Figure 4.10, a greater extent of hemolysis was observed when the volume of blood was increased to 30 µL. After 50 µL of 1x HSS was added as a final wash, red blood cells (RBC) were washed up the membrane and occupied a much larger portion of the membrane strip. For instance, the RBCs that were previously retained below 1.2 cm from the bottom of the membrane had gone up as far as 3.5 cm by the time all 1x HSS was fully absorbed. It should be noted that the images of the membranes that absorbed 50 and 70 µL whole blood respectively and were washed by 1xHSS do not represent the true distance which RBC traveled because the membrane were already fully saturated by the point whole blood filtration was completed . Based on these observations, it was determined that the optimal capacity for whole blood on FUSION 5 should be around 30 µL, and the length of FUSION 5 needed to complete filtration of 30 µL of whole blood ranged from 3 – 4 cm. The optimal length of the strip should be designed to reduce an excessive of wicking time and non-specific bindings. 32 Figure 4.10. Blood filtration/ migration on FUSION 5. Left: 10, 30, 50,70, 100 µL of whole blood was applied into a test tube, and a strip was placed into the tube and allowed to absorb blood, respectively. Right: 10, 30, 50, 70µL of whole blood was applied into a test tube, and a strip was placed into the tube and allowed to absorb blood, followed by 50 µL 1xHSS, respectively. 4.223 PVP/PES membrane The PVP/PES had promising results as potential whole-blood filtering sample pad materials. The performances of the two separator membranes were compared. Membrane X slightly outperformed membrane SG as the better separator membrane with a shorter wicking time. Therefore, membrane X was used as the primary separator membrane from this point on. To determine the best collector membrane, several membrane characteristics were particularly important to be investigated upon, first being the plasma capillarity and capacity. Ideally, a good collector membrane should exhibit a good capillary attraction force for plasma10. In the experiment where dyed plasma was allowed to migrate up the collector membrane, it was observed that the membranes CS and NX had similar appearances, indicative of similar membrane characteristics in terms of plasma capillarity and membrane capacity. The results also demonstrated that for membrane CS and NX, the length of membrane determined based on manufacture’s specifications was 33 approximately enough to accommodate the volume of plasma expected. The volume of plasma/whole blood applied and the approximated length of membrane needed was tabulated in the Table 4.3. Membrane C/Q-I on the other hand, demonstrated poorer capillarity. On average, it took almost twice as long for C/Q-I to fully absorb the same volume of plasma as the other two membranes, and a longer membrane was needed to accommodate the same volume of plasma. Table 4.3 Whole blood and plasma capacity of PVP/PES membranes (X,CS,C/Q-I, NX). Corresponding distance of migration measured when known volumes of plasma or blood was allowed to saturate the membrane. In addition, a direct comparison between the membranes in the blocked (with 1%BSA, 0.02% PVP, 0.1% Sodium Casein, 1X TBS, 0.002% Tween20, 0.05% sodium azide) and unblocked state showed that blocking had a slight effect on plasma capillarity after the membranes were blocked. The wicking time was approximately the same for 34 blocked and unblocked membranes, but the membrane appearances seemed to show that blocking the membrane might allow a more evenly plasma flow (Figure 4.11) a) b) c) Figure 4.11 Plasma Capillarity Diagrams on NX, CS, and C/Q-I. Top: 10 µL of dyed plasma (with SRB) was allowed to migrate up the membrane strip NX, CS, C/Q-I respectively. Bottom: 12 µL of dyed plasma (with SRB) was allowed to migrate up the membrane NX, CS, C/Q-I respectively. Membranes were unblocked on the left, and blocked with a solution containing 1%BSA, 0.02% PVP, 0.1% Sodium Casein, 1X TBS, 0.002% Tween20, 0.05% sodium azide on the right. 35 Subsequently, the extent of hemolysis and RBC leakage of the collector membrane were examined. Each membrane design comprised of a 2 cm long collector membrane and a 2 cm long separator membrane. The combinations being investigated are membrane X plus membrane NX, membrane X plus membrane C-Q/I m and membrane X plus membrane CS, all in which membrane X was positioned below the collector membrane to absorb and separate plasma out of whole blood. Among the three selected membrane filters, the combination of membranes X and membrane NX seemed most promising for our purpose when 30 and 40 µL of whole blood sample was applied onto PVP/PES through capillary flow (Figure 4.12). Whole blood was fully absorbed by the filter membrane within 15 and 25 minutes, respectively. The filtrate that passed beyond the sample pad showed no signs of hemolysis and RBC leakage when only whole blood was applied. However, after 50 µL 1x HSS was added, trace of RBC leakage was observed at the filter edge between the collector membrane and the nitrocellulose membrane on one of the two membranes tested. On the other hand, membrane strips on which 40 µL of whole blood was applied and filtered could not accommodate all the HSS buffer that was added afterwards. Furthermore, hemolysis and RBC leakage became visible after washing. Both observations were indications that volumes of blood and buffer applied exceeded the limit for capacity set by the length of the membrane. 36 Figure 4.12 Whole blood filtration on Millipore Hi-Flow Plus 120 membrane using X(2cm) plus NX(2cm) as the sample pad. Whole blood (30µL or 40 µL), with or without the addition of 50 µL 1xHSS, was allowed to saturate the membrane through capillary force. The other two combinations were also examined. The combination of membranes X plus CS was less satisfactory due to a greater extent of hemolysis, which was obvious even at 30 µL of whole blood (Figure 4.13). Since whole blood did not saturate the collector membrane before the membrane was washed with 1xHSS, hemolysis was probably not due to deficient capacity but poorer porosity gradient. Because a previous screening has shown that membrane C/Q-I had a much slower wicking time and a smaller capacity for plasma, it was not likely that the combination of membranes X and C/Q-I would be as competent as the combination of membranes X and NX. As expected, it took 25 minutes for the filtration of 30 µL of whole blood to complete for test with membranes X and C/Q-I as the sample pad (Figure 4.14), 10 minutes more than what was needed for NX and CS. In conclusion, among the three collector membranes, membrane 37 NX was proven to be most suitable collector membrane for blood filtration of 30 µL of whole blood based on the above observations. Figure 4.13 Whole blood filtration on Millipore Hi-Flow Plus 120 membrane using X(2cm) plus CS (2 cm) as the sample pad. Whole blood (30µL or 40 µL), with or without the addition of 50 µL 1xHSS, was allowed to saturate the membrane through capillary force. Figure 4.14 Whole blood filtration on Millipore Hi-Flow Plus 120 membrane using X(2cm) plus CQ-I(2cm) as the sample pad. Whole blood (30µL or 40 µL) was allowed to saturate the membrane through capillary force. 38 4.3 Optimization of Whole Blood Filtration and Signal to Noise Ratios 4.31 PVP/PES membranes After determining that the membrane combination of X+NX was the most suitable PVP/PES membrane as whole blood filter, the performance of the sample pad at various blood concentrations was explored further by using whole blood samples spiked with PBS buffered insulin. In previous literatures, whole blood was diluted to facilitate the filtration process10,19, 20. However, since the volume capacity was limited, there existed a trade-off between possible loss of analyte and sensitivity of the test. To determine a range for optimal dilution percentage, test results with 50% diluted blood and 95% diluted blood were compared. When 50% diluted blood was filtered and analyzed, positive results were obtained on all membranes except for the negative control (Figure 4.15). However, the color of the filtrate above the collector membrane indicated evident cell hemolysis that was not observed earlier with undiluted whole blood samples (Figure 4.12). Also, leakage of RBC originated from blood cells slipping though the outer collector membrane edge was observed on one of the membranes. The time necessary to obtain a clear signal on each LFA tests was 35 to 40 minutes, because the migration of liposomes through the sample pad congested with RBCs became difficult and excessively time consuming (Figure 4.15). All 50 µL of the 1xHSS was fully taken up by the membrane after 40-50 minutes, indicating that the membrane’s full capacity could be as much as 90 µL total volume. 39 Figure 4.15. Filtration and analysis of 50% diluted whole blood spiked with insulin at 5µg/mL (30µL total). Sample was filtered and analyzed on a 4.5mm wide test strip (Millipore Hi-Flow 120 Plus) with the sample pad made up of membranes X (2cm) and NX (2cm). Anti-insulin #E86802M (500 µg mL-1) was immobilized in a test line (3.3 cm from the proximal end of the membrane card), and 1 mg mL-1 anti-streptavidin was immobilized in a control line (3.8 from the proximal end). 50% diluted whole blood spiked with insulin at 5µg/mL (30µL total ) was placed in a glass test tube (12 x 75 mm). The test strip was then inserted into the test tube. After the blood was absorbed (about 20 min), 5µL of liposomes conjugated to anti-insulin and 5µL of liposomes conjugated to anti- streptavidin were added to the same glass tube and allowed to be absorbed, followed by the addition of 30µ L of 1xHSS. Once the 1xHSS was completely absorbed onto the strip (about 15 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively. Extended experiments were conducted to optimize the tests involving whole blood filtration. The Canadian patent that Spectral Diagnostics, Inc., had filed revealed that removal of the collector membrane prior to analysis was necessary to maintain the full sensitivity of the test. The sample pad, the collector membrane in particular, made use of a porosity gradient to trap RBCs. In other words, the pores were largest at the top of the membrane, but were gradually becoming smaller as the flow of RBCs proceeded. As a result, there was an asymmetric accumulation of RBCs at the sample pad. This explained how liposomes and other components of the test were still able to pass through the filter which was supposedly clogged with blood cells. To determine if improvements could be achieved by removal of the sample pad, the sample pad was disposed after an additional washing step of 10 µl 1xPBS was included after whole blood was completely absorbed and filtered. When only the separator pad was removed, hemolytic activities were significantly reduced which can be observed from the clearer background on the 40 nitrocellulose membrane. At the same time, RBC leakage through the outer edge of the collector membrane was also reduced while the signal intensity was retained. When the sample pad was completely removed, neither hemolysis nor cell leakage was observed, indicating that this being the most effective method of minimizing undesirable factors (Figure 4.16). For both shortened membranes, the wick time was reduced by at least 10 minutes as expected. Figure 4.16. Filtration and analysis of 30µL of 50% diluted whole blood spiked with 5 µg/mL insulin. Sample was filtered and analyzed on a 4.5 mm wide test strip (Millipore Hi-Flow 120 Plus) with the sample pad made up of membranes X (2 cm) and NX ( 2cm). Anti-insulin #E86802M (500 µg mL-1) was immobilized in a test line (3.3 cm from the proximal end of the membrane card), and 1 mg mL-1 anti-streptavidin was immobilized in a control line (3.8 from the proximal end). 50% diluted whole blood spiked with insulin at 5µg/mL (30µL total ) was placed in a glass test tube (12 x 75 mm). The test strip was then inserted into the test tube. After blood was absorbed (about 20 min), the sample pad or partially removed. 5 µL of liposomes conjugated to anti-insulin and 5µL of liposomes conjugated to anti- streptavidin were then added to the same glass tube and allowed to be absorbed, followed by the addition of 50 µL of 1xHSS. Once the 1xHSS was completely absorbed onto the strip (about 5 min), the test strips were taken out. The signal intensity of the test line was estimated visually qualitatively. The experiment was repeated with 95% diluted blood spiked with insulin. Based on the previous observations, it was concluded that the maximum volume of whole blood and buffer that can be accommodated on the membrane of the fixed dimension were approximately 30 µL and 85 µL respectively. However, wicking time increased with the membrane’s level of saturation. In order to determine if the wicking time was affected when more concentrated blood samples were filtered, two controls were set up with 41 whole blood diluted in 1xPBS only (4.17). Surprisingly, the time necessary for the control line (anti-streptavidin) to become visible was about 10 minutes shorter than before when more diluted blood was filtered. This indicated that the sample pad was more congested earlier when the same volume of more diluted blood was analyzed. Also, the time needed for liposomes to migrate towards the control line after 5 µL PBS was added between blood and liposome additions, was notably shorter than that without any PBS washing. This again demonstrated that removing the PBS washing step might allow a faster flow rate. Figure 4.17. Filtration and analysis of 95% diluted whole blood in PBS. 30µL sample was filtered and analyzed on a 4.5mm wide test strip (Millipore Hi-Flow 120 Plus) with the sample pad made up of membranes X (2cm) and NX (2cm). Anti-insulin #E86802M (500 µg mL-1) was immobilized in a test line (3.3 cm from the proximal end of the membrane card), and 1 mg mL-1 anti-streptavidin was immobilized in a control line (3.8 from the proximal end). 95% diluted whole blood spiked with insulin at 100µg/mL (30µL total ) was placed in a glass test tube (12 x 75 mm). The test strip was then inserted into the test tube. After the blood was absorbed (about 20 min), 5µL of liposomes conjugated to anti-insulin and 5µL of liposomes conjugated to antistreptavidin were added to the same glass tube and allowed to be absorbed, followed by the addition of 50µ L of 1xHSS. Once the 1xHSS was completely absorbed onto the strip (about 5 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively. Next, 95% diluted blood spiked with insulin was filtered and analyzed using the same set up. The washing step with 1xPBS following whole blood filtration was omitted based on previous observations. The presence of hemolysis was not visible, nor was any RBC leakage. The nitrocellulose membrane portion had a much clearer background when compared with the results before with 50% diluted blood samples. For both membranes, final signals were obtained within 30 minutes. But interestingly, the collector membrane, 42 now packed with a greater number of RBCs with more concentrated blood, did not allow for a uniform flow of liposomes across the full width of the membrane. Instead, liposomes was observed to pass beyond the collector membrane and reach the test and control line in a spiked fashion (Figure 4.18 and Figure 4.19). In other words, a greater concentration of liposomes reached the center of the line, creating a dot-shaped signal instead of an even line. The test line, which was higher up, was affected more because the spike was sharper at the point of contact. To facilitate liposome migration, part of the sample pad was again removed. Unfortunately, results were only slightly improved when only the separator membrane was removed. Nevertheless, removing part of the sample pad reduced the loss of liposomes in the separator membrane region, which could be seen in Figure 4-14b. However, when additional 20 µL of 1xHSS was added to flush the liposomes further up, the membrane seemed to have reached its capacity for buffer, based on the observation that the absorbent pad could not draw up any more fluid. Regrettably, the experiments could not be conducted further with the entire sample pad removed due to a limited quantity of whole blood sample. However, based on what was found, it can be speculated that removing the collector membrane would most likely improve the appearance of the signals. 43 Figure 4.18. Filtration and analysis of 30 µL of 95% diluted whole blood in PBS spiked with insulin. Sample insulin concentration was 5µg mL-1. Sample was filtered and analyzed on a 4.5mm wide test strip (Millipore Hi-Flow 120 Plus) with the sample pad made up of membranes X (2 cm) and NX (2 cm). Anti-insulin #E86802M (500 µg mL-1) was immobilized in a test line (3.3 cm from the proximal end of the membrane card), and 1 mg mL-1 anti-streptavidin was immobilized in a control line (3.8 cm from the proximal end). 95% diluted whole blood spiked with insulin at 100 µg/mL (30µL total ) was placed in a glass test tube (12 x 75 mm). The test strip was then inserted into the test tube. After the blood was absorbed (about 20 min), 5µL of liposomes conjugated to antiinsulin and 5µL of liposomes conjugated to anti- streptavidin were added to the same glass tube and allowed to be absorbed, followed by the addition of 50 µL of 1xHSS. Once the 1xHSS was completely absorbed onto the strip (about 5 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively. Figure 4.19. Filtration and analysis of 30 µL of 95% diluted whole blood spiked with insulin. 30 µL insulin (5 µg/mL) spiked blood was filtered and analyzed on a 4.5mm wide test strip (Millipore Hi-Flow 120 Plus) with the sample pad made up of membranes X (2 cm) and NX (2 cm). Anti-insulin #E86802M (500 µg mL-1) was immobilized in a test line (3.3 cm from the proximal end of the membrane card), and 1 mg mL-1 antistreptavidin was immobilized in a control line (3.8 cm from the proximal end). 95% diluted whole blood spiked with insulin at 100µg/mL (30 µL total ) was placed in a glass test tube (12 x 75 mm). The test strip was then inserted into the test tube. After the blood was absorbed (about 20 min), the separator membranes were disposed. 5 µL of liposomes conjugated to anti-insulin and 5 µL of liposomes conjugated to anti- streptavidin were added to the same glass tube and allowed to be absorbed, followed by the addition of 50 µL of 1xHSS. Once the 1xHSS was completely absorbed onto the strip (about 5 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively. Previously, Thorslund et al. had found that PVP/PES filter also provided by Spectral Diagnostics, experienced RBC leakage that could not be improved by dilution10. 44 Their observation did not necessarily contradict with what was reported in this study. First of all, Thorslund et al. did not specify which type of PVP/PES membrane was used in their design. As shown earlier, the three types of collector membranes (C/Q-I, CS, and NX) showed different extent of RBC leakage induced. See Figure 4.12, 4.13, and 4.14 for direct comparisons. Second of all, it was observed in this study that, diluting the blood down did not help reduce the RBC leakage. On the contrary, RBC leakage was greater when more diluted blood was filtered. Therefore, the approach that Thorslund et al. adopted might have had the exact opposite effect, which could explain why the approach failed. A possible explanation for these observations might be that the porosity gradient construction in the PVP/PES filters were specifically designed to allow the membranes to be only partially clogged with RBCs, leaving enough room for the migration of other components of the tests such as liposomes to flow through the membrane. Conversely, addition of buffer and saline solution tend to saturate membranes more than whole blood, and simultaneously weakens the effectiveness of the porosity gradient. 4.32 FUSION 5 It has been previously determined that FUSION 5 cannot be applied in a traditional lateral flow assay with direct protein immobilization. On the other hand, since it could be used as a whole blood filter, its whole blood filtering properties were examined by integrating it with Millipore Hi-Flow Plus 120 membrane cards. In a similar set up to the membrane strips comprised of PVP/PES membranes, FUSION 5 was used instead as sample pad to perform whole blood filtration. Based on previous observations, as shown in Figure 4.10, it was determined that the length necessary for a 4.5 mm wide 45 membrane to filter 30 µL of whole blood should be in the range of 3 to 4 cm. To find the optimal length membrane length, various lengths were integrated onto the membrane cards. PBS-buffered insulin spiked whole blood (95% diluted) was filtered and analyzed with these membranes. Results are shown in Figure 4.20. RBC leakage and hemolysis were most evident when Fusion 5 was at 2 cm, indicating that the sample pad was over its capacity after 1xHSS added, as expected. As the length of FUSION 5 was increased to 3 cm, RBCs were found to be retained further away from the reaction membrane. At 4 cm, FUSION 5 seemed to be able to retain most of it RBC even after flushing with xxx, producing cleaner backgrounds, but RBC leakage was still apparent. Figure 4.20. Filtration and analysis of 30 µL 95% diluted whole blood spiked with insulin. Final insulin concentration was 5 µg/mL. Sample was filtered and analyzed on a 4.5mm wide test strip (Millipore Hi-Flow 120 Plus) with the sample pad made up of FUSION 5 at various lengths. Anti-insulin #E86802M (500 µg mL-1) was immobilized in a test line (3.3 cm from the proximal end of the membrane card), and 1 mg mL-1 anti-streptavidin was immobilized in a control line (3.8 from the proximal end). 95% diluted whole blood spiked with insulin at 100µg/mL (30µL total ) was placed in a glass test tube (12 x 75 mm). The test strip was then inserted into the test tube. After the blood was absorbed (about 10 min),5µL of liposomes conjugated to anti-insulin and 5µL of liposomes conjugated to anti- streptavidin were added to the same glass tube and allowed to be absorbed, followed by the addition of 50µ L of 1xHSS. Once the 1xHSS was completely absorbed onto the strip (about 5 min), the test strip was taken out. The signal intensity of the test line was estimated visually qualitatively. 46 Unlike PVP/PES membranes which had a tightly packed pore structure, FUSION 5’s open pore nature allows more concentrated blood to be filtered without RBCs clogging the sample pad at a faster speed. Nevertheless, at the same time, the larger pore size (11 µm) in FUSION 5 also allowed a small amount of RBC, which had a typical diameter of 6-8 µm, to leak through the sample pad, showing a greater extent of RBC leakage. 47 Chapter 5 –Conclusion and Future Work 5.1 Optimization of PVP/PES plasma separation membrane In conclusion, the commercial PVP/PES plasma separation membrane, the combination of membranes X and NX in particular, was found to be most suitable for the purpose of this study. It experienced the least degree of hemolysis and RBC leakage when used with 95% blood samples, and moderate hemolysis and blood cell leakage with 50% blood samples. In this study, the dimension of the sample pad was not investigated in detail. In order to achieve a more efficient whole blood filtration, future efforts should be focused on finding the right dimensions of the membrane that can accommodate a maximal volume of sample while keeping hemolysis and RBC leakage low and wicking time short. On the other hand, pre-treatment of whole blood samples are also worth looking into. As earlier observations showed, there exists a trade-off between the extent of hemolysis activities/RBC leakgage and signal smoothness, as the whole blood concentration varies. Chemical methods that can be used to facilitate whole blood filtration, if any, should be investigated and combined with physical approaches, i.e. filtration via sample pad, to speed up the process. In addition, future work should also be focused on testing the reproducibility of the model and the feasibility of obtaining semiquantitative or quantitative results22. 5.2 Evaluation of FUSION 5 using Latex Beads Due to the lack of latex beads during the time of the experiments, full set-ups with FUSION 5 following the manufacture’s protocol could not be completed. Even though 48 FUSION 5 was less satisfactory in terms of whole blood filtration when compared to the PVP/PES membranes, it was still nonetheless an innovative, multifunctional matrix with great potential for lateral flow assay applications. The application and model design based on FUSION 5 should be pursued and explored further. In addition, other potential membrane materials, such as Pall’s highly asymmetric BTS-BP membrane are all potential sample materials worth of investigation. 49 References 1 O’Farrel, Brendan, Jeff Bauer. “Developing highly sensitive, more reproducible lateral flow assays. Part 2: New challenges with new approaches.” IVD Technology. July, 2006 2 Schalkhammer, Thomas G.M.,eds. Analytical Biotechnology (Methods and Tools in Biosciences and Medicine). Basel,CH: Birkhäuser Basel, 2002. 3 Wong, Raphael C., Harley Y. Tse. Drugs of Abuse: Body Fluid Testing (Forensic Science and Medicine). Totowa, NJ: Humana Press, 2005. 4 Edwards Katie A., Antje J. Baeumner. “Liposomes in analyses.” Talanta. Vol 68, Issue 5, pp 1421-1431. 2006. 5 Qian, Shizhi, Haim H.Bau. “Analysis of lateral flow biodetectors: competitive format.”Analytical Biochemistry. Vol 326, Issue 2, pp 211-224. 2004. 6 Parkash, Om, Avnish Kumar, Richa Pandey, Astha Nigam, and Bhawneshwar Kumar Girdhar. “Performance of a lateral flow test for the detection of leprosy patients in India.” Journal of Medical Microbiology. Vol 57, Issue 1, pp 130 – 132. 2008. 7 Sehgal, S.C., P. Vijayachari, A.P. Sugunan, T.Umapathi. “Field application of Lepto Lateral flow for rapid diagnosis of leptospirosis.” Journal of Medical Microbiology. Vol 52, pp 897 – 901. 2003. 8 Chan, Cangel P.Y., Ka Wai Sum, Kwan Yee Cheung, Jan F.C. Glatz, John E.Sanderson, Albrecht Hempel, Matthias Lehmann, Ilka Renneberg, Reinhard Renneberg. “Development of a quantitative lateral-flow assay for rapid detection of fatty acid-binding protein.” Journal of Immunological Methods. Vol 279, Issue 1-2, pp 91-100. 2003. 9 Bunce, Roger A., Gary Thorpe, Jan Hall, Philip Poissant. Lateral Flow Filter Devices for Separation of Body Fluids From Particulate Materials. Spectral Diagnostics, Inc, assignee. Patent 5,916,521. 29 Jun. 1999. 10 Thorslund, Sara, Oliver Klett, Fredrik Nikolajeff, Karin Markides, Jonas Bergquist. “A hybrid poly(dimethylsiloxane) microsystem for on-chip whole blood filtration optimized for steroid screening.” Biomedical Microdevices. Vol 8, Issue 2, pp 7379. 2006. 11 Jones, Kevin, Klaus Hochleitner. “Assay development: Changes in the development of rapid assays since 1995.” IVD Technology. April, 2005 50 12 Edwards, K. A. “Conjugation of Proteins to COOH-tagged Sulforhodamine B encapsulating Liposomes.” 2007. 13 Edwards, K. A. “Preparation of HanoRapid Nitrocellulose Membrane.” 2007. 14 Edwards, K. A. “Preparation of Anti-streptavidin Lateral-flow Assay Membrane.” 2007. 15 Edwards, K. A. “Lateral Flow Assay on Anti-StAv membranes.” 2007. 16 Edwards, K. A. “Lateral Flow Antibody Assay - Hand Preparation of Membranes on HanoRapid.” 2007. 17 Wang, Joseph, Alfredo Ibáñez, Madhu Prakash Chatrathi. “On-Chip Integration of Enzyme and Immunoassays: Simultaneous Measurements of Insulin and Glucose.” J. Am. Chem. Vol 125, Issue 28, pp 8444-8445. 2003. 18 Ho, Ja-an A., Shi-Chin Zeng, Ming-Ray Huang, Hung-Yi Kuo. “Development of liposomal immunosensor for the measurement of insulin with femtomole detection.” Analytica Chimica Acta. Vol 556, pp 127-132. 2006. 19 Jong, J.de, R. G. H. Lammertink,M. Wessling. “Membranes and Microfluidics: a review.” Lab Chip, Issue 6, pp 1125 - 1139. 2006. 20 Crowley, Timothy A., Vincent Pizziconi. “Isolation of plasma from whole blood using planar microfilters for lab-on-a chip applications.” Lab Chip, Issue 5, pp 922 - 929. 2005. 21 Kim, So Young., Myung Ja Choi. “Preparation and Characterization of digoxin antibody and its application to immunoassays: comparison of performance characteristics between enzyme immunoassay and immonostrip test.”Microchemical Journal. Vol 65, pp 209-219. 2000. 22 Anonymous. Rapid Lateral Flow Test Strips: Considerations for Product Development, Millipore (Lit. no. TB500N00 Rev. B). 2008. 51