Protein manipulation
advertisement

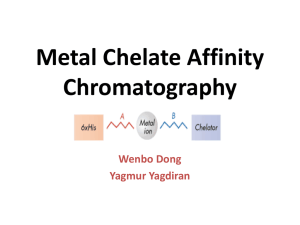
Protein Manipulation 김경규 (성균관대 의학과) Overview of Biochemical Experiments (characterization) Immunochemistry Antibody production Physicochemical characterization: MW (electrophoresis=EP, mass), size (EP, gel-filtration, centrifuge), purity (EP, chromatography), concentration (Bradford assay, spectroscopy), PI: electrofocusing (Partially) Purified biological materials Functional assay Identification (2D EP, Western, mass), enzymatic assay & kinetic study (EP, UV-Vis, fluorescence, isotope), P-P binding assay (EP, pull-down, migration assay, ITC, Biacore, gel-filtration, blotting), P-D binding assay (bandshift assay, footprinting assay), Chemical modification Structure study 2nd structure (CD, IR), 3rd structure (EM, X-ray, NMR), distance (fluorescence), conformational change (fluorescence, CD, UV-VIS, IR) Why do you need proteins? (amount & purity) 1.How are you going to prepare the large amount of protein? - from tissue or using a recombinant system. 1.How are you going to purify protein? - using their biochemical and biophysical characters. 1.How are you going to maintain their function (activity): keep their fold in native state. What do you need to know? -Protein, what is it? -Structure & folding -Biochemical and biophysical characters -Function : binding & catalytic activity -What factors affect the structure and function of protein? Purpose? Antibody production Function study Structure study Production Synthesis (protein or peptide) Recombinant protein Endogenous protein Purity Partially purified Maintenance Short term storage > 95 % Medium term storage Long term storage Protein Chemistry Protein → Amino Acids 20 Amino Acids Protein folding Alanine 7.2% Cysteine 1.9% Aspartic acid 5.3% Glutamic acid 6.3% Phenylalanine 3.9% Glycine 7.2% Histidine 2.3% Isoleucine 5.3% Valine Lysine 5.9% Leucine 9.1% Methionine 2.2% Asparagine 4.3% Proline 5.2% Glutamine 4.3% Arginine 5.1% Serine 6.8% Threonine 5.9% 6.6% Tryptophane 1.4% Tyrosine 3.2% Protein stability and activity Factors affecting the stability and activity of proteins: temperature, pH, protease, microbial contamination, protein concentration, organic solvents, buffer, salt etc. (denaturation, preteolytic digestion, chemical modification, adsorption) Purpose? Antibody production Function study Structure study Production Synthesis (protein or peptide) Recombinant protein Endogenous protein Purity Partially purified Maintenance Short term storage > 95 % Medium term storage Long term storage Expression systems Heterologous Protein Expression (host, vector) 1. Bacteria: E. coli, Bacillus etc (plasmid) 2. Yeast: Saccharomyces cerevisiae, Pichia pastoris (plasmid) 3. Insect cell: Spodoptera frugiperda (Sf), Trichoplusia ni TN-368 (High 5) (Baculovirus) 4. Animal cell: CHO cells, 293 cells etc (virus or plasmid) 5. In vitro translation: E. coli extract, wheat germ extract, rabbit recticulocyte (transcription & translation, plasmid) 6. Plant Type Cost Time Yield Folding Comment bacteria 1 1 2 5 Yeast 2 2 4 3 Insect cell 3 2 3 2 Animal cell 4(5) 5 5 1 In vitro (cell free) 5(4) 1 1 2 Prokaryotic expression - Why E. coli? Simple and inexpensive. (total 50 % of E.coli proteins can be the heterologous proteins. - Why other systems? expressed proteins might not be active (folding problem). - Components * Regulatory elements for transcription control (promoter) => strong, tightly regulated, induction * Translation initiation and termination => RBS or Shine-Dalgarno sequence (must be located optimally from the start codon) => TAA is preferred as a stop codon (less prone to read-through) * MCS site for insertion * Marker for selection Ori Marker Prokaryotic expression: low expression level or insoluble protein - Vector and host selection * protease-deficient host * rare codon t-RNA * secondary structure of RNA near translation initiation site * copy numbers of plasmid * promoter selection - Expression condition: * growth temperature * inducer concentration * secretion (concentration and disulfide bond), * co-expression with chaperone - Fusion protein (+detection and purification) pET system (unique features) - Tight control of basal expression level (DE3, pLysS, pLysE) - Host selection - Various fusion tags for purification (Novagen Catalogue) (Novagen Catalogue) Prokaryotic expression - procedures Primer design PCR Purification of PCR product Restriction enzyme treatment Gel extraction of the restricted PCR product Ligation of PCR product with digested vector Transformation into BL21(DE3) Colony selection Culture & IPTG induction Cell harvest Expression test in SDS-PAGE Purification XhoI NotI HindIII SalI F1 origin EcoRI BamHI NcoI TEV site KpnI NheI NdeI NcoI KanR pVFT1L vector 5378bp lacI Insect cell expression Sf9 cells Plaques Transfection Virus purification infection (Modified from Invitrogen Catalogue) Expressed proteins Sf9 cells transfected with b-gal Mammalian cell expression system : CHO cells YGI 100 ug DHFR 5 ug YPI YPI No NTPs CHO DHFR - Add MTX (DHFR inhibitor) DHFR+ YGI+ YPI 100-500X DHFR 100-500X YGI YPI YPI YPI YPI Grow cells and collect conditioned serum free media Mammalian cell expression system: 293 or Hella cells YGI Virus infection or transfection YPI YPI 293 cells Selection By marker YPI Colony selection YPI YPI YPI YPI YGI+ Grow cells and collect conditioned serum free media In vitro translation (cell-free protein synthesis) (Modified from Invitrogen Catalogue) Heterologous protein expression (host, vector) 1. Bacteria: E. coli, bacillus etc (plasmid) 2. Yeast: Saccharomyces cerevisiae, Pichia pastoris (plasmid) 3. Insect cell: Spodoptera frugiperda (Sf), Trichoplusia ni TN-368 (High 5) (Baculovirus) 4. Animal cell: CHO cell, 293 cell etc (virus, plasmid) 5. In vitro translation: E. coli extract, Wheat germ extract, rabbit recticulocyte (transcription & translation, plasmid) 6. Plant Type Cost Time Yield folding comment bacteria 1 1 2 5 insoluble Yeast 2 2 4 3 Insect cell 3 2 3 2 Glycosylation issue Animal cell 4(5) 5 5 1 Glycosylation issue In vitro (cell free) 5(4) 1 1 2 Good for toxic proteins Purpose? Antibody production Function study Structure study Production Synthesis (protein or peptide) Recombinant protein Endogenous protein Purity Partially purified Maintenance Short term storage > 95 % Medium term storage Long term storage Protein purification References : Protein purification , Robert Scopes, Springer-Verlag Modern experimental biochemistry, Rodney Boyer, Benjamin Cummings Protein purification techniques, Simon Roe, Oxford GE healthcare catalogue ,. General consideration for purification 1. Maximize the yield and minimize the cost and time 2. Separate proteins using their physicochemical characters 3. Remove major impurity first 4. Use the most effective method first 5. Use the most expensive method last 6. Make your protein active Protein handling: 1. Principle: keep protein active and stable 2. Factors: contamination, denaturation, preteolytic digestion, chemical modification, adsorption 3. Methods: - Mild condition (pH, temp. surface) – avoid the denaturation - Concentration (avoid low and high conc.) - Stability and degradation (low temp., protease inhibitor, sodium azide, short exposure to high temp.) - Stabilizing agent such as glycerol - Reducing condition - No freeze & thaw Protein Purification – general scheme Protein overexpression or raw materials Cell Paste Cell disruption and debris separation Crude extract Pretreatment of samples or low-resolution chromatography Partially purified (1st) protein Protein separation using chromatography Partially purified (2nd) protein Purity < 95 % Protein separation using high-resolution chromatography Purified protein Function studies Chromatography - - Principle : samples can be interact with a mobile phase (gas or liquid) & a stationary phase (column) Chromatography – separate the molecules by adsorption, partition and/or path (charge) differences - Preparation and analytical chromatograpies Resolution 1. Factors: size and rigidity of resin, packing, low diffusion, protein concentration, selection of resin, optimal flow rate, capacity 2. Performance: High & Low - pump & resin (pressure) HPLC (High performance Liquid Chromatography) 3. For high resolution column chromatography - Homogeneous small beads (homogenous packing) - high resistance to liquid flow (high pressure operation) - short theoretical plate height (high resolution) - decreasing diffusion (no line broadening, improving resolution) - porous bead – high capacity, size independent experiment (big enough for the penetration of proteins or large molecules) - smaller volume General procedures of column chromatography 1. 2. 3. 4. 5. 6. 7. (Packing the column) Pre-equilibrium Loading the column Washing the column Eluting the column Collecting the eluting components Detecting the eluting components General considerations for the column chromatographic separation 1. Sample volume, amount of protein. resolution, time, 2. Column size & packing, flow rate & chromatographic system must be considered for maximum resolution in a short time 3. Resin (matrix) - Condition: rigidity, nonspecific interaction, chemical stability, open pore, small and regular size - Cellulose, dextran, agrose, polyacrylamide or their mixture Column chromatography 1. Chromatography on the basis of the physical property of proteins : gel-filtration 1. Chromatography on the basis of chemical property of proteins: ion-exchange, hydrophobic, reverse-phase, charge-transfer 2. Chromatography on the basis of the biochemcal activity of proteins : affinity Gel-filtration - - Principle: separate proteins using the size difference Consideration: size of protein, column (height/width of column), sample volume, media, pore size & buffer (solubility of protein & non-specific interaction) Choice: Gel-filtration & desalting together, choice for the next or previous step of ion-exchange Elution: buffer Gel-filtration Matrics Applications of gel-filtration columns 1.Separation 2.Desalting: change the buffer 3. Size determination Ion-exchange - Principle: separate proteins using the charge difference - Consideration: Matrix, functional residues - cation (CM, SP, S) or anion (DEAE, QAE, Q), buffer pH & salt in buffer, PI of proteins - Choice: usually used for the first step after initial treatment, however high resolution ion exchange columns are also recommended in the final stage - Elution: salt elution or pH elution Effect of pH on surface charge … Elution … Affinity chromatography - Principle: separate proteins using the binding affinity difference to ligands - Choice: best choice if you are able to make the affinity column harboring a high selectivity to your protein (105~1011 M) - Consideration: matrix (large surface area, less non-specific binding), ligand, linker (spacer, steric hinderance), immobilization (cynogen bromide can be used for attaching Lys residues to the matrix) - General ligands: Metal binding, GST, intein, MBP - Specific ligands: ATP, NADH, DNA, RNA, antibody & protein or chemical ligand - Elution: salt or specific chemicals Charge transfer chromatography - Metal affinity chromatography: … Affinity chromatography : Ligand Affinity chromatography : activation Hydrophobic interaction chromatography Hydrophobic interaction column: - Principle: separate proteins using the hydrophobicity difference - Consideration: matrix, hydrophobicity of proteins, ligand, salt conc. in buffer - Choice: choice for the next step of ion exchange or AMS ppt. - Elution : salt elution … Refolding 1. Principle : folding, no aggregation 2. Consideration: pH, concentration, reducing condition, additives, chaperones 3. Practice (1) purification of the inclusion body (2) solubilization (by chaotrophic agents such as GdHCl, urea or detergents) (3) refolding by reducing the concentration of denaturants one step-dialysis, step-wise dialysis, gel-filtration dilution, reverse dilution, Mixing, solid phase refolding (4) characterization of the refolded proteins (activity or function) Purpose? Antibody production Function study Structure study Production Synthesis (protein or peptide) Recombinant protein Endogenous protein Purity Partially purified Maintenance Short term storage > 95 % Medium term storage Long term storage Storage Storage 1. Principle : maintaining their stability and activity 2. Factors affecting the stability of proteins: temperature ,pH, proteases, microbial contamination, concentration, organic solvents, buffer, salt etc.. 3. Practice (1) Short term storage : keep proteins at low temperature (with protease inhibitors) (2) Medium term storage : keep proteins at low temperature with sodium azide (in the case of putting them in a buffer) or in water. (3) Long-term storage : keep proteins in deep freezer (solution or dried): - aliquot protein solution in the amount needed for short term experiments - do not thaw and freeze again. Purpose? Antibody production Function study Structure study Production Synthesis (protein or peptide) Recombinant protein Endogenous protein Purity Partially purified Maintenance Short term storage > 95 % Medium term storage Long term storage Why do you need proteins? (amount & purity) 1.How are you going to prepare the large amount of protein? - from tissue or using a recombinant system. 1.How are you going to purify protein? - using their biochemical and biophysical characters. 1.How are you going to maintain their function (activity): keep their fold in native state. What do you need to know? -Protein, what is it? -Structure & folding -Biochemical and biophysical characters -Function : binding & catalytic activity -What factors affect the structure and function of protein?