5 Protein Primary Structure - School of Chemistry and Biochemistry
advertisement

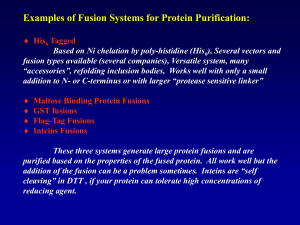
Revised 2/14/2014 Biochemistry I Dr. Loren Williams Chapter 5 Proteins: Primary Structure A protein is one or more large polymers consisting of linear chains of amino acids. Proteins perform a vast array of functions within living organisms, including: (i) catalyzing chemical reactions, (ii) regulating reactions, (iii) transport and sequestration (iv) pores and channels (v) structure (stiffness and rigidity), (vi) tensile strength, (vii) compartments and adhesives (viii) signaling molecules and hormones (ix) optical devices Proteins differ from one another primarily in their sequence of amino acids, which usually results in the folding of the protein into a specific three-dimensional structure that determines its activity. Gelatin is a colorless, tasteless, water-soluble derivative of collagen of cow or pig. Made from bones, hides and connective tissues. Collagen doesn't dissolve in waterThe body parts are ground and treated with strong acid or strong base, then boiled, then dried and ground to a powder. Gelatin is contained in jello, skittles, gummy bears, starbursts, marshmellows... There are 2,598,960 combinations of 5 cards in a 52 card deck. There are 400 billion stars in the Milky Way. There are a around a septillion stars (1024) in the universe. There are 10130 possible protein sequences of 100 amino acids in length (20100) Table 5-1 Bovine Insulin Insulin is a hormone made of polypeptide (i.e., it is a protein) that is produced by the pancreas. Insulin central to regulating carbohydrate and fat metabolism in mammals. Table 5-5 part 1 Table 5-5 part 2 Figure 5-21 Figure 5-22 Decide on a protein source: Whale myoglobin is easy to get (in Japan). Human transcription factors are low copy number and can only be obtained by over-production by recombinant methods (usually in E. coli). Develop methods to detect the protein (absorbance at 280 nm, catalytic activity, gel mobility, fluorescence, etc). Myoglobin was the first protein whose three-dimensional structure was determined by x-ray diffraction. The experiments used a lot of purified protein, which at that time could not be produced by recombinant methods. Myoglobin is the primary oxygen-carrying protein of muscle tissues. Myoglobin is particularly abundant in marine mammels, allowing the animals to hold their breaths for long periods. Break open (lyse) the cells. Keep the cell lysate on ice Add protease inhibitors Treat tubes and surfaces with albumin Control the pH with buffers illustration of cell lysis from biofreak (http://bio-ggs.blogspot.com/) Figure 5-5 You hope that your over-expressed protein remains soluble in the E. coli. But sometimes it will form inclusion bodies. Inclusion bodies are dense particles of aggregated foreign protein caused by high-level expression. Methods for cell disruption Osmotic Pressure Detergents Beadbeating [tiny hard beads and intense agitation by stirring or shaking] Sonication [Ultrasound (20–50 kHz)] French Press [A piston forces a pressurized sample through a needle valve, causing shear stress and decompression] Bovine erythrocytes under osmotic stress Page 97 Separate your protein from other cellular components. Ammonium sulfate precipitation Proteins can be separated from a mixture on the basis of relative hydrophilicity by gradually increasing the concentration of ammonium sulphate. Add ammonium sulfate and pellet cellular proteins by spinning in a centrifuge. Discard the supernatant, resuspend the protein and redisolve with detergents. Then remove the ammonium sulfate and detergent by dialysis. Chromatography Flow a solution containing the protein through a column containing resin. Proteins can interact selectively with various types of resins, and can thus be separated by the time required to pass the column, or by the conditions required to elute the protein from the column. Size exclusion chromatography: The resin contains pores. Smaller proteins enter pores and migrate more slowly. Hydrophobic interaction chromatography (reverse phase): The resin contains a hydrophonic material (silica modified with C18, etc). Elute by decreasing the aqueous content of the solvent and increasing the ethanol. Ion exchange: The resin is charged. Anionic resins are positively charge retain negatively charged proteins. Cationic resins hare negatively charged and retain positively charged protein. Protein can be eluted by high salt. Size Exclusion Chromatography Size Exclusion Chromatography Figure 5-7 Ion Exchange Chromatography Figure 5-6 Affinity Tagging and Protein Over-Expression Fuse the tag to your protein: There are many different tags available. - To express and purify a protein use MBP (maltose binding protein), GST (gluthatione Stransferase), His (hexahistidine)…. - To study cellular localization or detect your protein easily, use fluorescent tag like GFP. Green Fluorescent Protein Maltose Binding Protein: The vector pMAL (a plasmid) is designed to create maltosebinding protein (MBP) fused to any target protein. A gene or open reading frame is inserted into a restriction site of the vector polylinker, in the same translational reading frame as the malE gene (encoding maltose-binding protein). The fusion protein produced in E. coli is generally stable and can be purified by amylose affinity chromatography (cheap and easy). After purification the fusion protein the protease Factor Xa separating the target protein from the MBP. Pmal System (MPB tag): SDS-polyacrylamide gel electrophoresis of fractions from the purification of MBPparamyosin-ΔSal. Lane 1: Protein Ladder (NEB #P7703 ). Lane 2: uninduced cells. Lane 3: induced cells. Lane 4: purified protein eluted from amylose column with maltose. Lane 5: purified protein after Factor Xa cleavage. Lane 6: paramyosin fragment in flow-through from second amylose column. absorption fluorescence Figure 5-4 Figure 5-9 staining The enzyme-linked immunosorbent assay (ELISA) is a test that uses antibodies and color change to identify a substance. Figure 5-3 Table 5-2 Figure 5-8 Figure 5-11 Figure 5-12 Box 5-1 Figure 5-13 Figure 5-13 part 1 Figure 5-13 part 2 Page 106 Page 107 Page 107 Table 5-3 Figure 5-14 Figure 5-14 part 1 Figure 5-14 part 2 Figure 5-14 part 3 Figure 5-15 Figure 5-15 part 1 Figure 5-15 part 2 Figure 5-16a Figure 5-16a part 1 Figure 5-16a part 2 Figure 5-16b Figure 5-17 Figure 5-18 Figure 5-19 Table 5-4 Figure 5-20 Figure 5-23 Figure 5-24