B. cereus
advertisement

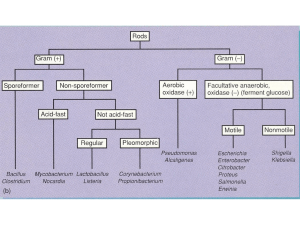
BACILLUS CEREUS 黃顯宗 Content INTRODUCTION OCCURRENCE IN FOOD AND ENVIRONMENT CHARACTERISTICS AND TAXONOMY SPORE AND GERMINATION ISOLATION AND ENUMERATION TYPING CONTROL VIRULENCE FACTORS Syndromes of B. cereus Food Poisoning Phospholipase and Sphingomyelinase Cereolysin Haemolysin BL Nonhaemolytic enterotoxin Enterotoxin T Cytotoxin K Detection of enterotoxins Emetic Toxin Bioassays of Enterotoxins and Emetic Toxin CONCLUSIONS INTRODUCTION The Genus Bacillus was established in 1872 with B. subtilis as type species. B. cereus was added fifteen years later Several accounts of food poisoning attributed to members of the genus Bacillus appeared in the European literature before 1950 INTRODUCTION An accumulating number of reports implicate both B. subtilis and B. licheniformis as potential food poisoning agents. The pattern of their repeated occurrence in association with episodes of food poisoning suggests a significant involvement However, application of the standard toxintesting methods used for B. cereus to isolates of B. subtilis and B. licheniformis associated with gastrointestinal illness have so far failed to indicate what mode of pathogenic action these organisms might have. OCCURRENCE IN FOOD AND ENVIRONMENT B. cereus has a wide distribution in nature, frequently isolated from soil and growing plants, but it is also well adapted for growth in the intestinal tract of insects and mammals It has been isolated from foods that were not involved in foodborne illness outbreaks. It is also present in the stools of 14 to 15% of healthy humans It is frequently isolated from milk and dairy products. In milk, B. cereus causes a defect known as 'bitty' cream or sweet curdling. It is found in rice, rice products, oriental dishes and ingredients OCCURRENCE IN FOOD AND ENVIRONMENT A variety of foods have been implicated in food-poisoning Emetic syndrome caused by B. cereus is highly associated with rice and rice products OCCURRENCE IN FOOD AND ENVIRONMENT B. cereus was isolated from 9, 35, 14 and 48% of raw milk, pasteurized milk, Cheddar cheese and ice cream samples, respectively In a local study, B. cereus occurred in 17% of fermented milks, 52% of ice creams, 35% of soft ice creams, 2% of pasteurized milks and pasteurized fruit- or nut-flavored reconstituted milks, and 29% of milk powders, mostly in fruit- or nut-flavored milk mixes (Wong et al., 1988a). B. cereus was found in 71.4% and 33.3% in spring and in autumn samples of full-fat milk in mainland China, respectively, and the average count among the positive samples was 11.7 MPN/ml (Zhou et al., 2008). Dried milk products and infant food are known to be frequently contaminated with B. cereus, 261 samples of infant food distributed in 17 countries were collected and 54% were contaminated with B. cereus reaching levels from 0.3 to 600/g (Becker et al., 1994). OCCURRENCE IN FOOD AND ENVIRONMENT Chinese 'take-out' foods appear to be particularly vulnerable to B. cereus infection and it has been shown that suspensions (2%) of seed flours and meals from diverse botanical origins were found to be excellent sources of nutrients for growth (Beuchat and Ma-Lin, 1980) OCCURRENCE IN FOOD AND ENVIRONMENT Of 433 honey samples collected in Argentina, 27% yielded B. cereus isolates and 14% yielded other species of Bacillus. The Argentinian B. cereus isolates were compared with isolates recovered from honey from other countries using rep-PCR fingerprinting with primers BOX, REP and ERIC, restriction fragment length polymorphism analysis of a 16S rRNA gene fragment (16S rRNA PCR/RFLP), and morphological and biochemical tests. Results showed a high degree of diversity, both phenotypic and genotypic among the isolates of B. cereus (Lopez and Alippi, 2007). OCCURRENCE IN FOOD AND ENVIRONMENT The B. cereus isolates from food are highly toxigenic. All the isolates from local dairy products lysed rabbit erythrocytes; 98% showed verotoxicity, 68% showed cytotonic toxicity for CHO cells (Wong et al., 1988a). In another study of 136 strains of B. cereus isolated from milk and cream, 43% and 22% showed toxicity to human embryonic lung cell when the isolates were cultured in brain heart infusion and milk, respectively (Christiansson et al., 1989). In milks, B. cereus growed rapidly and produced cytotonic and cytotoxic toxins (Wong et al., 1988b). Toxin production of B. cereus in milk at low temperature was also evaluated (Christiansson et al., 1989). OCCURRENCE IN FOOD AND ENVIRONMENT For the B. cereus isolated from seafood, 48% isolates produced both the hemolysin BL (HBL) and nonhemolytic (NHE) enterotoxins, and 94% and 50% produced NHE or HBL toxins, respectively. Only one B. cereus isolate possessed the cereulide synthetase gene, ces (Rahmati and Labbe, 2008). OCCURRENCE IN FOOD AND ENVIRONMENT The enterotoxin genes hblA, hblC, hblD, nheA, nheB and nheC occurred in B. cereus isolates from full-fat milk products with frequencies of 37.0%, 66.3%, 71.7%, 71.7%, 62.0% and 71.7% respectively Nine B. thuringiensis isolates were also identified from six pasteurized milk samples, and most of them harbored six enterotoxic genes and the insecticidal toxin cry1A gene. The single B. mycoides isolate harbored nheA and nheC genes (Zhou et al., 2008). CHARACTERISTICS AND TAXONOMY B. cereus is a Gram-positive, motile, facultative, aerobic sporeformer. Dimensions of vegetative cells are typically 1.01.2 μm by 3.0-5.0 μm. The ellipsoidal spores are formed in a central or paracentral position without swelling the sporangium. The organism does not ferment mannitol and has a very active phospholipase (lecithinase) system. B. cereus is keyed as citrate(+), arabinose (-), Gram (+), aerobic sporeformer. CHARACTERISTICS AND TAXONOMY CHARACTERISTICS AND TAXONOMY CHARACTERISTICS AND TAXONOMY The bacilli tend to occur in chains; the stability of the chains determines the form of the colony, which varies greatly in different strains. The G+C content of the DNA is reported to be 32-33 moles % (determined by Tm) and 33-37 moles (analysis). CHARACTERISTICS AND TAXONOMY 分類特性 CHARACTERISTICS AND TAXONOMY . Phylogenetic analysis shows that the B. cereus group of bacteria are closely related group (Fig. 1) (Stenfors Arnesen et al., 2008). Conjugative behavior shows that these Bacillus species are closely related. CHARACTERISTICS AND TAXONOMY Plasmids have been identified in B. cereus. Plasmids of molecular weight ranged from 1.6 to 105 MDa. Bacteriocin production could be attributed to a 45 MDa plasmid (pBC7), and tetracycline resistance to a 2.8 MDa plasmid (pBC16) SPORE AND GERMINATION B. cereus produces elliptical shaped endospore with dominant central position, no distended sporangium. The spore when liberated from the sporangium is encased in a loose fitting exosporium. On germination the spore coat undergoes rapid lysis while the vegetative cell is emerging. Since spores of B. cereus may survive heat processing, spore germination is important in B. cereus study. GERMINATION STEPS Once the initial 'trigger reaction' has been activated, germination continues in the absence of the inducer. After the 'trigger' steps, the various spore properties are changed sequentially in the following order: loss of heat resistance, release of dipicolinic acid (DPA) and Ca2+ into the medium, increase in spore stainability, beginning of phase darkening and decrease of the optical density of spore suspension as cortex peptidoglycan is hydrolyzed and the products released to the medium Finally, the onset of metabolic activity as measured by oxygen uptake. Role of trypsin-like enzyme The germination of B. cereus spore is partially prevented by several inhibitors of trypsin-like enzymes (leupeptin, antipain, and tosyl-lysine-chloromethyl ketone) A synthetic substrate of trypsin also inhibited germination. A crude extract of germinated B. cereus spores contained a trypsin-like enzyme whose activity is sensitive to germination-inhibitory compounds such as leupeptin, tosyl-arginine-methyl ester, and tosyl-lysine-chloromethyl ketone. Spore suspensions exposed to the above inhibitors under germination conditions lose only part of their heat resistance and some 10-30% of their dipicolinic acid content (Boschwitz et al., 1983). SPORE AND GERMINATION Inactivation of B. cereus spores during cooling from 90C occurs in two phases, one phase occurs during cooling from 90 to 80C; the second occurs during cooling from 46 to 38C. No inactivation occurs when spores are cooled from a maximum temperature of 80C. Why? SPORE AND GERMINATION Germination of B. cereus spores is more extensive in rice than in trypticase soy broth at <15C and is generally more extensive for diarrheal strains in either medium than emetic strains Germination of B. cereus spores was also inhibited by the growth of lactic acid bacteria or the organic acids produced (Wong and Chen, 1988). SPORE AND GERMINATION B. cereus spores germinate in inosine or in l-alanine as sole germinants They require both GerI and GerQ germinant receptors for germination in inosine as the sole germinant, whereas the GerL receptor is responsible for most of the response to lalanine as the sole germinant, with a smaller contribution from the GerI receptor Confirmation of outbreak B. cereus strains of the same serotype should be present in the epidemiologically food, feces and/or vomitus of the affected persons. Or Significant numbers (>105 CFU/g) of B. cereus of an established food poisoning serotype should be isolated from the incriminated food, or feces, or vomitus of the affected persons. or Significant numbers (>105 CFU/g) of B. cereus should be isolated from the incriminated food, together with detection of the organism in the feces and/or vomitus of the affected persons. ISOLATION AND ENUMERATION Mannitol egg yolk polymyxin agar (MYP) is usually recommended. Polymyxin is the selective agent, and egg yolk and mannitol are differential agents Typical colonies are rough with a violet-red background, surrounded by white precipitated egg yolk. ISOLATION AND ENUMERATION Polymyxin pyruvate egg yolk mannitol bromothymol blue agar (PEMBA) is a modified selective agar, and also contains polymyxin and egg yolk. Pyruvate is added to reduce the size of colonies. The authors state that this medium is superior in detecting lecithinase-negative strains of B. cereus, weak and negative egg yolk reacting strains also developed typical colored colonies, grey to turquoise blue, and the color turns to a peacock blue color after 48 h (Holbrook and Anderson, 1980). ISOLATION AND ENUMERATION Two new chromogenic plating media (CBC and BCM) were compared with two standard selective plating media (PEMBA and MYP) recommended by food authorities for isolation, identification and enumeration of B. cereus authors addressed that the new chromogenic media represent a good alternative to the conventional standard media (Fricker et al., 2008). SEROTYPING Developed at the Food Hygiene Laboratory, England Based on the established typespecificity of the flagellar (H) antigen The scheme currently comprises a 'routine set' of 28 agglutinating antisera raised against prototype strains In approximately 90% of outbreaks the causative serotypes can be established. SEROTYPING Most of the outbreaks associated with a vomiting-type syndrome, foods, clinical specimens or both yielded H-serotype 1 only. But only a few of diarrheal-type outbreaks yielded serotype 1 only PHAGE TYPING Phage typing scheme has been developed for B. cereus. By using 12 bacteriophages, 10 Myoviridae and 2 Siphoviridae phages isolated from sewage, were employed (Ahmed et al., 1995). http://viralzone.expasy.org/all_by_species/140.html http://viralzone.expasy.org/all_by_species/142.html TYPING Biotypes, fatty acid profiles, and restriction fragment length polymorphisms of a PCR product (PCR-RFLPof the cereolysin AB gene) were compared for 62 isolates of the B. cereus group originated from various foods. The isolates were clustered into 6 biotypes, 10 fatty acid groups, or 7 PCR-RFLP clusters and these schemes may be used in tracking the origination of B. cereus strains (Schraft et al., 1996). amplified fragment length polymorphism (AFLP) method the chromosome DNA is digested by HindIII, ligated to adapters (ACG GTATGC GAC AG and GAGTGC CATACGCTGTCTCGA amplified by PCR using primers (GGTATGCGACAGAGCTTA, GGTATGCGACAGAGCTTC, G GTATGCGACAGAGCTTG and GGTATGCGACAGAGCTTT) (Ripabelli et al., 2000). AFLP method Multilocus sequence typing (MLST) Primers were designed for conserved regions of housekeeping genes, and 330- to 504-bp internal fragments of seven such genes were sequenced for all strains. adk (encoding adenylate kinase), ccpA (catabolite control protein A) ftsA (cell division protein) glpT (glycerol-3-phosphate permease) pyrE (orotate phosphoribosyltransferase) recF (DNA replication and repair protein), and sucC (succinyl coenzyme A synthetase, beta subunit) Multilocus sequence typing (MLST) Primers were designed (Table 5) for conserved regions of housekeeping genes, and 330- to 504bp internal fragments of seven such genes, adk (encoding adenylate kinase), ccpA (catabolite control protein A), ftsA (cell division protein), glpT (glycerol-3-phosphate permease), pyrE (orotate phosphoribosyltransferase), recF (DNA replication and repair protein), and sucC (succinyl coenzyme A synthetase, beta subunit) were sequenced for all strains. The number of alleles at individual loci ranged from 25 to 40 (Table 6), Multilocus sequence typing (MLST) Multilocus sequence typing (MLST) The number of alleles at individual loci ranged from 25 to 40 dN/dS ratio: nonsynonymous/synonymous substitution rate ratios Multilocus sequence typing (MLST) a total of 53 allelic profiles or sequence types (STs) were distinguished Multilocus sequence typing (MLST) Analysis of the sequence data showed that the population structure of the B. cereus group is weakly clonal. In particular, all five B. anthracis isolates analyzed had the same ST. The MLST scheme has a high level of resolution and should be an excellent tool for studying the population structure and epidemiology of the B. cereus group MLST Phylogenetic analysis was performed on a total of 296 strains for which MLST sequence information is available (MLST database (http://pubmlst.org/bcereus/) three main lineages--I, II, and III--within the B. cereus complex were identified With few exceptions, all food-borne isolates were in group I. MLST horizontal gene transfer (HGT) of toxinencoding genes among various strains determined CONTROL Heated B. cereus did not grow at 10 and 50C or in a medium with pH 4.0. Decreasing pH values and increasing levels of sodium chloride decreased growth rate and increased the lag phase of B. cereus. pH 4.5 was unable to prevent the growth of heated spores in a meat substrate with 0.5% NaCl at 12C. The combination of pH </=4.5, NaCl concentration >/=1.0% and temperatures </=12C was sufficient to inhibit B. cereus growth after heat treatment at 90 C for 10 min, for at least 50 days in nutrient broth and in meat extract (Martinez et al., 2007). CONTROL The combination of mild acidification (pH 5.0) and refrigeration (</=8C) inhibited B. cereus growth for at least 60 days in vegetable substrates. Psychrotrophic strains of B. cereus were inhibited in carrot broth by heating at 90 C for 7.5 min, if the broth was refrigerated at a temperature of 8 C or lower. If the vegetable product was exposed to temperatures of mild abuse (12 C), it was necessary to implement a more drastic heat treatment (90 C for 30 min) (Valero et al., 2003). CONTROL A combination of electrolyzed water and 1% citric acid exhibits synergistic effect on the inactivation of B. cereus vegetative cells and spores (Park et al., 2009). Growth and germination of B. cereus are inhibited by lactic acid bacteria and the organic acids produced by these bacteria, e.g. acetate, formate, and lactate. Spores of B. cereus are more resistant to these organic acids (Wong and Chen, 1988). Electrolyzed water http://www.ewatersystems.com.au/e-water/electrolyzedwater/how-does-it-work/ CONTROL A combination of electrolyzed water and 1% citric acid peracetic acid-based disinfectant ozone Pulsed Electric Field (PEF) technology High pressure around 300 MPa Several antimicrobial wine recipes, each consisting of red or white wine extracts of oregano leaves with added garlic juice and oregano oil are bactericidal Essences of vegetables Carvacrol, a natural antimicrobial compound present in the essential oil fraction of oregano (奧立岡)and thyme (百里香) http://www.stuartxchange.com/Oregano.html http://www.bbc.co.uk/food/thyme bacteriocin enterocin AS-48 ( 20-35 μg/ml )against the toxicogenic psychrotrophic strain B. cereus Enterotoxin production at 37C was also inhibited. Heat sensitivity of endospores increased markedly in food samples supplemented with enterocin AS-48 (Grande et al., 2006) Synergistic activity of epsilon-poly-L-lysine and nisin A VIRULENCE In addition to causing foodborne illness, B. cereus is also capable of causing mastitis, systemic infection, gangrene, meningitis in immunocompromised children (Gaur et al., 2001), respiratory tract infections (Gray et al., 1999), and other clinical problems (Weber, 1988). VIRULENCE Usually, two types of B. cereus foodborne diseases occur, the diarrhoeal and the emetic types Cell culture assays Cell culture assays measuring the cytotoxic activity of cell-free culture supernatants is now more commonly used to detect the presence of B. cereus diarrheal toxins, and these give a good indication of the cytotoxic potential of B. cereus strains. VIRULENCE FACTORS B. cereus produces a large number of secreted cytotoxins and enzymes that may contribute to diarrhoeal disease the identity of the enterotoxin(s) is still a controversial topic The three cytotoxins are currently considered the aetiological agents of B. cereus diarrhoeal foodborne disease haemolysin BL (Hbl) nonhaemolytic enterotoxin (Nhe) cytotoxin K. Hbl and Nhe are related three-component toxins, while the singlecomponent CytK belongs to the family of b-barrel pore-forming toxins. VIRULENCE FACTORS In addition, several other protein cytotoxins, haemolysins and degradative enzymes have been described that may potentially contribute to the pathogenicity of B. cereus diarrhoeal disease. These include cereolysin O, haemolysin II, haemolysin III, InhA2 (metalloprotease) and three phospholipases C Phospholipase and Sphingomyelinase Phospholipase and Sphingomyelinase were known to be toxic, but now they have been demonstrated to be nontoxic, and some of the hemolysins associated with them are marginally toxic (Beecher et al., 2000) Phospholipase Phospholipase (Lecithinase) similar to the α-toxin of Clostridium perfringens. Phospholipase of B. cereus is resistant to inactivation at 45C and also resistant to trypsin inactivation. It is a small metalloprotein (MW 23,000 Da) containing two zinc atoms per molecule of enzyme (El-sayed and Roberts, 1983). Phospholipase activity can be determined by observing zones of turbidity on agar plate containing 1% egg yolk. Production of phospholipase is regulated under the transcriptional regulator, PlcR, which controls proteins, of which 22 were secreted in the extracellular medium and 18 were bound or attached to cell wall structures (membrane or peptidoglycan layer). These regulated proteins are related to food supply (phospholipases, proteases, toxins), cell protection (bacteriocins, toxins, transporters, cell wall biogenesis) and environment-sensing (Gohar et al., 2008) Sphingomyelinase It is a protein of between 41,000 and 23,300 Da, depending on the method of analysis used requires divalent cations for activity can be assayed as follows: culture filtrate is mixed with phosphate-buffered saline and TNPAL-sphingomyelin solution (N-wtrinitrophenyl aminolauryl sphingosyl phosphoryl choline). Cereolysin purified to apparent homogeneity by using ammonium sulfate fractionation, hydrophobic chromatography with AH-Sepharose, isoelectric focusing, and gel filtration. The active form of the toxin had an isoelectric point (pI) of 6.6, and the molecular weight of 55,000 Cereolysin is a cholesterol-dependant cytolysins, containing two halfcystine residues. In the absence of dithriothreitol, partial spontaneous oxidation resulted in the formation of an oxidized form of the toxin. cereolysin is a thiol- or SH-activated hemolysin (cytolysins) similar to the streptolysin O (produced by Streptococcus pyogenes), pneumolysin (Streptococcus pneumoniae), and listeriolysin (Listeria monocytogenes). They are apparently cross-react in neutralization and immunodiffusion tests. They are activated by thiol-reducing agents Haemolysin BL HBL is a tripartite toxin produced by B. cereus, and it has been highly purified and established to be a diarrheal toxin by the ligated rabbit ileal loop assay. HBL is identical to the toxin purified (Thompson et al., 1984) by performing Western blots and immunodiffusion assays Haemolysin BL The B. cereus enterotoxin is capable of causing fluid accumulation in ligated rabbit ileal loops, altering the vascular permeability or rabbit and guinea-pig skin and killing mice when injected intravenously The enterotoxin is synthesized and released during the late logarithmic growth phase of the organism at an optimum temperature of 32-37C. Haemolysin BL The growth medium employed markedly affected the ability of a given strain of B. cereus to provoke a response. Brain Heart Infusion broth proved to be best for toxin production in small scale CA medium consisting of casamino acids, yeast extract and minerals supplemented with 1% glucose was shown to be optimum for fermenter production of B. cereus enterotoxin at 32C, controlled pH 8.0, and moderate stirring rate Haemolysin BL The enterotoxin is thermolabile and susceptible to protease inactivation Activity of enterotoxin may involve stimulation of adenylate cyclase-cAMP system with probable role in nongastrointestinal infections Haemolysin BL HBL is a unique and potent three component pore forming toxin composed of a binding component, B, and two lytic components, L(1) and L(2). Nucleotide and deduced amino acid sequences have been reported for all components (GenBank accession nos. L20441, U63928, AJ237785). The genes hblC (L2), hblD (L1), and hblA (B) are arranged in tandem in an operon with the promoter located upstream of hblC. Haemolysin BL Only Components B and L1 contain predicted transmembrane segments of 17 and 60 amino acid residues, respectively All three components in HBL are required for biological activity. HBL produces a unique discontinuous hemolysis pattern on blood agar. Hemolysis begins several millimeters from the edge of a colony or a well containing HBL, forming a ring-shaped clearing zone (discontinuous). Haemolysin BL HBL is dermonecrotic, increases vascular permeability in rabbit skin, and is cytotoxic to Chinese hamster ovary cells and retinal tissue both in vitro and in vivo HBL forms pores in eukaryotic cell membranes, with each of the components binding the membrane independently and reversibly A high degree of molecular heterogeneity exists in HBL from different strains Haemolysin BL Commercially available kit (BCET RPLA, Oxoid) is useful for detection of L(2) component of HBL, but detection of only one component is insufficient to give comprehensive view on HBL toxin producing strains as some strains produced only one or two of the three HBL components. Nonhaemolytic enterotoxin NHE is also a multi-component toxin Nonhaemolytic enterotoxin Various combinations of the individual NHE subunits possess some degree of biological activity, but maximal activity is achieved only when all components are present. N-terminal amino acid sequence similarity exists between L1 of HBL and the 39 kDa subunit of NHE, as well as between L2 and the 45 kDa subunit of NHE, suggesting similar functional roles in different B. cereus strains. Nonhaemolytic enterotoxin Both NheA and NheB appear to be present in culture supernatants in two forms with slightly differing sizes, where the smallest form represents a further processed variant of the largest form. The smallest forms of NheA and NheB lack 11 and 12 N-terminal amino acids, respectively, in addition to the 26 and 30 residues of their signal peptides Nonhaemolytic enterotoxin The nature of the cytotoxic activity of Nhe towards epithelial cells showed rapid disruption of the plasma membrane following exposure to Nhe, and formation of pores in planar lipid bilayers Nonhaemolytic enterotoxin Vero cell assay A, toxin B, control Nonhaemolytic enterotoxin (A)1.5 % human erythrocytes Enterotoxin T a single component enterotoxin and appears to possess biological activity similar to HBL and NHE. The authors were able to detect the enterotoxin T gene, bceT, in all ten strains tested by PCR, including some environmental isolates. However, no evidence exists that the 41 kDa enterotoxin T has been involved in any cases of B. cereus food poisoning. Cytotoxin K single-component protein toxins that are members of the family of β-barrel poreforming toxin This toxin family includes β-toxin of Clostridium perfringens and α-haemolysin of S. aureus CytK is a 34-kDa protein with dermonecrotic, cytotoxic and haemolytic activities, and shows similar cytotoxic potency towards cell cultures as Hbl and Nhe (Lund et al., 2000) Emetic Toxin Emetic toxin, cereulide, produced by B. cereus has been purified Optimum production occurs in a rice culture slurry incubated at 25-30C during the stationary growth phase of the organism Cereulide It is a small ring-formed dodecadepsipeptide with the structure [D-O-Leu-D-Ala-D-O-Val-D-Val]3 Its genetic determinant is plasmid-borne (EhlingSchulz et al., 2006). The peptide is 1,165 Da with a predicted pI of 5.52. Cereulide is hydrophobic and not easily solubilized in aqueous solutions and may be delivered to its target cells bound to or dissolved in carriers found in food (Schoeni and Wong, 2005). Detection of cereulide Two animal models, rhesus monkey (Macaca mulatta) and Asian musk shrew (Suncus murinus)(臭鼩 ), have been used for cereulide assays The HEp-2 vacuolation assay with colorimetric modifications is commonly used. In this assay, the mitochondrial swelling caused by cereulide appears as cytoplasmic vacuoles in HEp-2 cells. Paralysis of boar spermatozoa and changes in oxidation rates in isolated rat liver mitochondria have also been used as indicators of cereulide-induced toxicity. Measurement of oxygen consumption in these assays indicates that cereulide acts by uncoupling mitochondrial oxidative phosphorylation (Schoeni and Wong, 2005).