Gram-Positive & Gram-Negative Bacilli: Microbiology Overview
advertisement

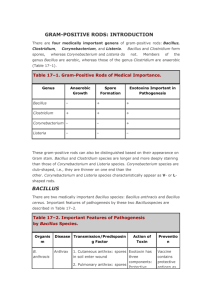
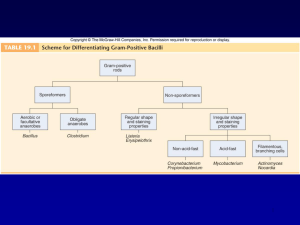
Non-Spore-Forming Gram-Positive Bacilli • Corynebacterium C. diphtheriae Disease Diphteria Opportunistic infections by other Corynebacterium species (dipheroids) Properties • Club-shaped also V- or L-shaped • Beaded appearance • Methachromatic granules (Albert staining) • Nonmotile • no capsule • Facultative anaerobic. • Classified in CNM group. Biotypes (based on colony shape, biochemical properties and virulence) Gravis Mitis Intermedius Belfanti Clinical finding • Common diphtheria (Nasopharyngitis) Incubation period of 2–5 days. Fibrinous exudate “pseudomembrane” Sore throat, fever, Enlargement of neck lymph nodes and neck edema. Irregulatory of cardiac rhythm, difficulties with vision, speech and swallowing. Corrosion of myelin sheaths in the central and peripheral nervous system leading to degenerating motor control Clinical finding • Cutanous diphtheria (a secondary infection) • Antibody production: Blocking the fragment B and so preventing entry into the cell. Transmission • Humans the only natural host • C. diphtheriae reside in the upper respiratory tract • Transmitted by airborn droplet • Infection at the site of a pre-existing skin lesion Pathogenesis • Invasivness • Exotoxin Invasivness • Cord factor A glycolipid inhibits eukaryotic cell oxidation. • Nuraminidase Removes N-acetyl nuraminic acid from musine membranes. Exotoxin (Encoded by gen tox from a temperate phage) Fragment B. Binding of the toxin Fragment A. Enzymatic activity A B Nicotinamide adenine dinucleotide phosphate (NAD) Exotoxin (A fragment) Nicotinamide ADP Reaction with EF2 Protein synthesis inhibition ADP-EF2 Testing immunity (Schick’s test) • Intradermal injection (0.1 mL): I. Cause inflammation (4-7 days later): No antitoxin in patient II. No inflammation: Antitoxin is present (Immune person) Laboratory diagnosis • Microscopic observation (differentiation from streptococcal and vansant nasopharyngitis) • Isolating the organism Loffler’s medium a tellurite plate Tinsdal medium • Demonstrating toxin production Animal inoculation Eleck test ELISA • PCR to detect tox gene Treatment • Tracheostomy in children (to prevent croup) • Antitoxin 20000-100000 unit (Intra muscular) • Penicillin or erythromycin Prevention • Vaccination A combination of diphtheria toxoid, tetanus toxoid, and killed pertusis organism. Given at 2, 4 an 6 months of age, with a booster at 1 and 6 years of age and then each 10 years afterward. (DPT or DT) The toxoid is prepared by treating the exotoxin with 0.3% formaldehyde. Listeria monocytogenes • • • • • Small rod like “chinese character” No capsule, Facultative aerobic. Tumbling movement. Movement in 25 c Growing in 4c Small and smooth colony on blood with a narrow zone of beta-hemolysis • Biochemical tests: Fermentation, Catalase + Oxidase + Disease • Meningitis and sepsis in 1. The fetus or newborn as a result of transmission across the placenta or during delivery. 2. Immunosuppressed adults (especially renal transplant patients) • The infected mother: asymptomatic or influenzalike illness/ Abortion Transmission • The organism is distributed worldwide in animals, plants and soil. • Transmission to human by contact with animals or their feces unpasteurized milk contaminated vegetables. Endogenously from gasterointestinal tract. Pathogenesis Internalin E-cadherin Phagocytosis into epithelial cells Forming filopods Phagocytiosis By macrophages and hepatocytes Inducing actin polymerization in cytoplasm Release from phagolysosome Phagolysosome formation (acidic condition) Lysteriolysin O secretion Lab. diagnosis • Microscopic observation: Diphtheroids • Isolation by culture: Blood and CSF samples on blood agar Colonies: Small, gray colonies with a narrow zone of beta hemolysis Treatment • Penicillin Resistant are rare Prevention • Cell-mediated immunity is active but no immunization • Limiting the exposure of immunosuppressed patients to potential sources Spore-forming gram-positive bacilli • Bacillus (Aerobic) B. antheracis, B.cereus • Clostridum (Anaerobic) C. tetani, C. botulinum, C. perfringens, C. difficile Bacillus anthracis • Disease Anthrax (common in animal but rare in humans). Properties • A large rod with square ends. • Frequently in chains • A unique anti-phagocytic capsule is composed of D-glutamate. • Non-motile (other members of the genus are motile.) Transmission • Spores persist in soil for years. Infection from animal products (hides, bristles and wool), contact with sick animal. • Portals of entry: skin, mucous membranes, and respiratory tract. Clinical findings • A typical lesion: A painless ulcer with black, necrotic eschar. Local edema. • Untreated cases progress to bacteremia and death. • Woolsorter’s disease (pulmonary anthrax) is a life threatening pneumonia (by inhalation of spores). Pathogenesis • Invasiveness • Exotoxin • Anthrax toxin, has 3 components: - Protective antigen - Lethal factor: In the presence of protective antigen is rapidly fatal for mice. The action is unknown - Edema factor (an exotoxin): An adenylate cyclase dependent on protective antigen for its binding and entry into the cell. Lab. diagnosis • Samples: Exudate, Blood, sputum. • Direct smear: Large rods in chains. Spores not seen in smears of exudate. • Culture and biological/biochemical tests (Sensitivity to penicillin (String of pearls test), Fermentation, gelatin hydrolysis, Motility) • No serological tests are useful Prevention • Preventing soil contamination • Sterilizing dead animals and animal products . • Protecting persons at risk of exposure with special clothes. • Vaccination with cell-free vaccine for persons at high risk. Treatment • Penicillin No resistant strain isolated Bacillus cereus • Motile • No capsule • Saprophyte Bacillus cereus • Disease Food poisoning Rare infections: Meningitis, Osteomyelitis, … • Transmission Spores on grains survive during steaming and rapid frying. Spore germinated when rice is kept warm. Portal of entry is the gastrointestinal tract. Pathogenesis • B. cereus produces 2 enterotoxins. Their actions is unclear. Clinical findings 1. Emetic syndrome A short incubation period (4 hours) with nausea and vomiting similar to staphylococcal food poisoning. 2. Diarrheal syndrome Involves a long incubation period (18 hours) with diarrhea and resembles clostridial gastroenteritis. Lab. diagnosis • Not usually done Treatment No antibiotic is given. Only symptomatic treatment Prevention Grains (specially rice) should not be reheated Clostridiums An aerobic bacteria Clostridiums tetani Peritricus flagella Terminal spore • Disease Tetanus (Lockjaw) Clinical findings • Incubation period: 4-5 days – several weeks • Violent muscle spasms in the site of infection and then jaw) • Lockjaw (trismus) due to rigid contraction of the jaw muscles, which prevents the mouth from opening: a characteristic known as “risus sardonicus”’. • Low blood pressure • Respiratory failure Neonatal tetanus Transmission Spores are widespread in soil. The portal of entry is a wound site. Germination of spores is favoured by necrotic tissue and poor blood supply in the wound. Pathogenesis • Tetanus toxin (tetanospasmin) It is carried intra-axonally (retrograde) to the central nervous system, where it binds to ganglioside receptors and blocks release of inhibitory mediators (e.g. glycine, Gammaaminobutiric acid) at spinal synapses leading to hyper reflection and spastic paralysis. Diagnosis • History of wound and clinical picyure • There is no microbiologic or serologic diagnosis. • Organisms are rarely isolated from the wound site. Treatment • • • • Antitoxin does have a low effect Penicillin Respiratory support Muscle relaxants Prevention • Immunization with toxoid in childhood (2, 4, 6, 12 months ages) and every 10 years thereafter. • When trauma occurs deeply: 1. Wound should be cleaned and debrided. 2. Tetanus toxoid booster should be given. 3. Tetanus immune globulin should be given. 4. Penicillin administered. Clostridium botulinum • Disease •Transmission •Pathogenesis •Clinical findings •Laboratory diagnosis •Treatment •Prevention Transmission • In soil ---> Alkaline vegetables/meat ---> canned/vacuum-packed ---> Spore germination ---> Toxin production ---> ingestion Pathogenesis • Botulinus toxin Observing from the gut ---> Carrying via the blood to peripheral nerve synapses ---> Blocking release of acetylcholine ---> Paralysis Clostridium perfringens • • • • • • • Disease: Gas Gangrene / Food Poisoning Transmission Pathogenesis Clinical findings Laboratory diagnosis Treatment Prevention Transmission • Soil, vegetative cells are members of normal flora in colon and vagina. • Is associated with war wounds. Pathogenesis and clinical findings • Alpha toxin: Lecithinase • Glycogen metabolism: Gas in tissues: Crepitation • Treatment Penicillin Wounds should be debrided H2O2 Crepitation Lab diagnosis • Smear of tissue and exudate samples: large positive rods. • Cultured anaerobically identified with fermentation reactions Food poisoning • Transmission: Soil and food. Survives cooking and grows to large numbers in reheated food, especially meat. • Pathogenesis: An enterotoxin (a protein in the spore coat) • Clinical findings: Incubation: 8-16 hours, then watery diarrhea with cramps and little vomiting. Resolves in 24 hours. Treatment and prevention • Treatment: Symptomatic – No antimicrobial drugs • Prevention: cooking well Clostridium difficile Disease Transmission Pathogenesis Clinical Finding Laboratory diagnosis Treatment Prevention Disease • Antibiotic-associated pseudomembranous colitis Transmission It is a part of normal flora of gasterointestinal tract (3%) Pathogenesis • Antibiotic (Clindamycin and ampicillin) supress drug-sensitive normal flora, allowing C. difficile to multiply: produce toxin. • Toxin mechanism is unclear Clinical findings • Diarrhea • Pseudomembranes (yellow-white plaques) on the colonic mucosa. • Visualised by sigmoidoscopy. Lab diagnosis • Toxin detectable in stool affecting on cell cultured cells. • Inhibition of cytotoxicity by specific antibody. Treatment • Withdrew the antibiotic • Oral vancomycin instead along with fluids.