File
advertisement

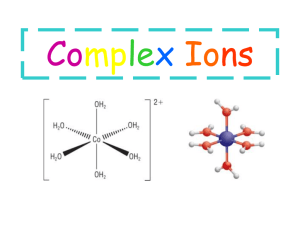
AP Net Ionic Equations AP equations are found in the free response section of the AP test. You will have 3 equations following by a question about the reaction. The equations are of mixed types. The sections is worth 15 points and is 10% of the free response grade. Free response is 50% of the total AP test grade. All AP equations “work.” In each case, a reaction will occur. These equations need to be written in net ionic form. All spectator ions must be left out and all ions must be written in ionic form. • Answer 3 equations that must be balanced. • Each equation is followed by a question. • 1 pt for reactants, 2 points for products, and 1 pt for each question. • Strong Acids are: Exception: concentrated sulfuric acid-keep together because it really is 97% H2SO4 and 3% water in the jug. • Strong Bases are: Weak acids and bases keep together • All molecular substances and nonsoluble compounds must be written together (not ionized!). Know your solubility rules!!! – Ca(OH)2 and Sr(OH) 2 are moderately soluble and can be written together or as ions. – Ba(OH)2 is soluble and Mg(OH)2 is insoluble. – CaSO4 and SrSO4 are moderately soluble and can be written together or as ions. – Weak electrolytes, such as acetic acid, are not ionized. – Solids and pure liquids are written together, also. – A saturated solution is written in ionic form while a suspension is written together. Double Replacement Two compounds react to form two new compounds. No changes in oxidation numbers occur. All double replacement reactions must have a “driving force” that removes a pair of ions from solution. • Manganese(II) nitrate solution is mixed with a sodium hydroxide solution • Excess hydrochloric acid is added to an aqueous solution of potassium sulfite • Hydrogen sulfide gas is bubbled through a solution of lead(II) nitrate • A solution of ammonium sulfate is added to a potassium hydroxide solution • Solutions of tripotassium phosphate and zinc nitrate are mixed • Gaseous hydrofluoric acid reacts with solid silicon dioxide. Single Replacement Rxns Treat like redox reactions. Reaction where one element displaces another in a compound. One element is oxidized and another is reduced. A + BC B + AC + charges replace + and – charges replace - • Active metals replace less active metals or hydrogen from their compounds in aqueous solution. • Active nonmetals replace less active nonmetals from their compounds in aqueous solution. Each halogen will displace less electronegative (heavier) halogens from their binary salts. Examples • A piece of aluminum metal is added to a solution of silver nitrate • Small chunks of solid sodium are added to water • Chlorine gas is bubbled into a solution of sodium bromide • Magnesium turnings are added to a solution of iron(III) chloride Anhydrides Anhydride means “without water.” Water is a reactant in each of these equations. Memorize the Rules Look for: 1. Oxides + H2O a. metallic oxide + H2O base b. nonmetallic oxide + H2O acid 2. Metal hydride + H2O metal hydroxide + H2 3. Group 1 and 2 nitride + H2O metal hydroxide + NH3 4. Phosphorus halide + H2O H3PO4 or H3PO3 + H(halide) acid Examples • Excess water is added to solid calcium hydride • Solid lithium hydride is added to water • Solid dinitrogen pentoxide is added to water • Solid potassium oxide is added to water • Phosphorus pentachloride solid is added to water • Methylamine gas is bubbled into distilled water Oxidation-Reduction Reactions • Redox reactions involve the transfer of electrons. The oxidation numbers of at least two elements must change. • Single replacement, some combination and some decomposition reactions are redox reactions. • To predict the products of a redox reaction, look at the reagents given to see if there is both an oxidizing agent and a reducing agent. • When a problem mentions an acidic or basic solution, it is probably redox. Common oxidizing agent Product formed MnO4- in acidic solution Mn2+ MnO2 in acidic solution Mn2+ MnO4- in neutral or basic solution MnO2 (s) Cr2O72- in acidic solution Cr3+ HNO3, concentrated NO2 HNO3, dilute NO H2SO4, hot, concentrated SO2 Common oxidizing agent Product formed Metal-ic ions Metal-ous ions Free halogens Halide ions Na2O2 NaOH HClO4 Cl- H2O2 H2O Common reducing agent Product formed Halide ions Free metals Sulfite ions or SO2 Free halogen Metal ions Sulfate ions Nitrite ions Nitrate ions Common reducing agent Product formed Free halogens, dilute basic solution Hypohalite ions Free halogens, conc. basic solution Halate ions Metal-ous ions Metal-ic ions H2O2 O2 C2O42- CO2 Examples • A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. • Hydrogen peroxide solution is added to a solution of iron(II) sulfate • Potassium permanganate solution is added to a solution of oxalic acid acidified with a few drops of sulfuric acid. • A piece of iron is added to a solution of iron(III) sulfate. • Solid sodium dichromate is added to an acidified solution of sodium iodide • Potassium permanganate is mixed with an alkaline solution of sodium sulfite • Copper (II) sulfide is oxidized by dilute nitric acid. • A solution of potassium iodide is added to an acidified solution of potassium dichromate. Acid-Base Neutralization Rxns Acids react with bases to produce water and salts. Examples Hydrogen sulfide gas is bubbled through a solution of potassium hydroxide • A solution of sodium hydroxide is added to a solution of sodium dihydrogen phosphate until the same number of moles of each compound has been added • Nitric acid is added to crystals of pure calcium oxide • Carbon dioxide is bubbled through a solution of sodium hydroxide Decomposition Reactions • Reaction where a compound breaks down into two or more elements or compounds. Heat, electrolysis, or a catalyst is usually necessary. Memorize the rules! 1. Metal Carbonate Metal oxide + CO2 2. Metal Chlorate Metal chloride + O2 3. Hydrogen peroxide water + oxygen 4. Ammonium carbonate ammonia + water + carbon dioxide 5. Sulfurous acid Sulfur dioxide + water 6. Carbonic acid carbon dioxide + water Examples • A solution of hydrogen peroxide is heated • Magnesium carbonate is heated • Potassium chlorate is heated in the presence of manganese dioxide • Solid ammonium carbonate is heated Addition Reactions • Two or more elements or compounds combine to form a single product. Memorize the rules! Most of these should already look familiar. 1. 2 cmpds form one compound 2. If excess use the higher oxidation number If limited use the lower oxidation number 3. Nonmetal oxide + water acid 4. Metal oxide + water base 5. Metal oxide + sulfur dioxide Metal sulfite 6. Metal oxide + carbon dioxide metal carbonate Examples • Magnesium oxide is added to a container of carbon dioxide gas • Solid calcium oxide is heated in the presence of sulfur trioxide gas • Calcium metal is heated strongly in nitrogen gas • The gases boron trifluoride and ammonia are mixed Combustion Reactions -Elements or compounds combine with oxygen. Memorize these rules. 1. Hydrocarbon + oxygen carbon dioxide + water 2. ammonia + oxygen NO + H2O if excess O2 NO2 + H2O 3. Nonmetal hydride + oxygen nonmetal oxide+ water 4. Nonmetal sulfide+oxygen nonmetal oxide + sulfur dioxide • Methane is burned in the presence of oxygen • Lithium metal is burned in air • Solid zinc sulfide is heated in an excess of oxygen • A piece of solid bismuth is heated strongly in oxygen Complex Ion Reactions • Complex ions are made up of a ____________ and a _____________. [Co(NH3)6]+3 is the complex ion NH3 is the ligand, Co is the metal • Possible metals: Cu/Zn/Ag/Cd/Fe/Al • Possible ligands: NH3, OH-1, SCN-1 • Magic number? Double charge to get magic number • • Exceptions to the trick: **[Al(OH)4]- **[Fe(SCN)]2+ Example: [Co(NH3)6] Cl3 NH3 is the ligand, [Co(NH3)6]+3 is the complex ion Common complex ions on AP equations **[Al(OH)4]- tetrahydroxoaluminate ion formed from: (Al or Al(OH)3 or Al3+ + OH-) [Ag(NH3)2]+ diamminesilver(I) ion formed from (Ag+ + NH3) [Zn(OH)4]2- tetrahydroxyzincate ion formed from (Zn(OH)2 + OH-) [Zn(NH3)4]2+ tetramminezinc ion formed from (Zn2+ + NH3) [Cu(NH3)4]2+ tetraminecopper(II) ion formed from (Cu2+ + NH3) [Cd(NH3)4]2+ tetraminecadmium(II) ion formed from (Cd2+ + NH3) **[Fe(SCN)]2+ thiocyanairon(III) ion formed from (Fe3+ + SCN-) [Ag(CN)2]- dicyanoargentate(I) ion formed from (Ag+ and CN-) Remember the following when writing complex ion reactions: 1. Acid to complex ion break it up 2. Acid to NH3 complex NH4+ + breakup complex ion 3. HCl + Ag complex AgCl + Breakup complex ion Examples • Concentrated (15M) ammonia is added in excess to a solution of copper (II) nitrate • Dilute hydrochloric acid is added to a solution of tetraminecopper (II) sulfate • A suspension of zinc hydroxide is treated with concentrated sodium hydroxide solution • Solid silver chloride is added to a concentrated solution of ammonia • Hydrochloric acid is added to a solution of dihydroxysilver bromide