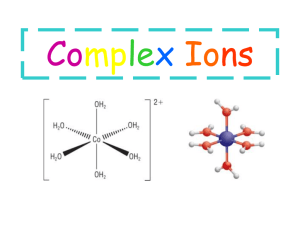
Pre- AP & NET IONIC EQUATIONS
advertisement

Frustrated student Frustrated Teacher • How do I fit it in my curriculum through out the year? • Why do the kids just not get it? • How can they learn so many reactions? Your approach may depend largely on the type of daily schedule your school follows. Block Schedule & Double Periods • Warm ups were net ionic equations • Time to discuss • Students have time to practice in class • Quiz students often… twice per week Single Periods & Modified Block • Warm ups took up to much time with discussion • Students do not have time in class to practice • Quizzes not as often once every week. I cannot change the school schedule. What do I do? Spend more time in pre-AP teaching reaction chemistry. • Nomenclature should be mastered in pre-AP. • This means knowing oxidation numbers. • Learning the most common polyatomic ions. POLYATOMIC ION CHARTS Quiz for weeks 3 & 4 Nitrate NO3- Carbonate CO32- Sulfate SO4 2- Chromate CrO42- Phosphate PO4 3-- Dichromate Cr2O72- Acetate Hydrogen sulfate HSO4- Hydroxide C2H3O2 – or CH3COOOH- Ammonium NH4+ Arsenate AsO 4 Oxalate C2O42- Permanganate MnO4- Hg22+ Peroxide O22- Di-mercury (mercury I) Bromate 3- BrO3- Quiz for weeks 5 & 6 will include all ions from this chart and previous chart Thiocyanate SCN- Hypochlorite ClO- Cyanide CN- Chlorite ClO2- Hydrogen phosphate Nitrite H 2PO4- Chlorate ClO3- NO2- Per chlorate ClO4-- Sulfite SO32- Arsenite AsO33- Phosphite PO32- Hydrogen Carbonate (bicarbonate HCO3- Monatomic Ions - Quizzes for week 7 – 18 will include all tables All group 1 metals 1+ Tin Sn2+ or Sn4+ All group 2 metals 2+ Zinc Zn2+ All group3 metals 3+ Silver Ag+ All group 16 (6) nonmetals All group 17 (7) nonmetals All group 15 (5) nonmetals except bismuth Iron 2- Copper Cu+ or Cu2+ 1- Cobalt Co2+ , Co3+, Co6+ 3Bi3+ Scandium Sc3+ Fe2+ or Fe3+ Nickel Ni2+ or Ni3+ Cr3+ , Cr2+ , or Cr6+ Manganese* Mn2+ or Mn4+ Chromium (there are more charges) Mercury II Hg2+ Mercury I Hg22+ • Students should also know how to calculate oxidation numbers elements (ions and compounds) • Identify the following for a redox reaction -element or ion oxidized -element or ion reduced -reducing agent -oxidizing agent - element or ion that loses electrons - element or ion that gains electrons If on the period system, you should reach nomenclature at the end of the 18th week. Students then take a nomenclature quiz every week. Work on reaction chemistry the last 9 weeks. Which one(s)? • Anhydrides • Combustion (Hydrocarbons) • Decomposition (simple) • Double replacement • Redox (Single replacement) Anhydrides can be taught with types of reactions as well as double, single replacement, and combustion. Taught as molecular equations The net ionic equations for double and single replacement can be taught after solutions. Of course, students will have to be introduced to solubility rules. This needs to be done in pre-AP (especially if not on a double or block period). Don’t re-invent the wheel…use the NMSI website for reactions or other printed resources. This is a great resource it also comes with a teachers edition with answers. The Ultimate Chemical Equations Handbook by George R Hague, Jr. and Jane D. Smith • Anydrides -Nonmetallic oxides plus water MgO + H2O → Mg(OH)2 -Metallic oxides plus water CO2 + H2O → H2CO3 -Metallic hydrides plus water NaH + H2O → Na+ + OH- + H2 - Group 1 plus water Na + H2 O → Na + + OH- + H2 • Anydrides -Nonmetallic oxides plus water CO2 + H2O → H2CO3 Solid magnesium oxide is added to water MgO + H2O → Mg(OH)2 -Metallic oxides plus water Gaseous carbon dioxide is bubbled through water CO2 + H2O → H2CO3 • Anydrides -Group I in water solid sodium is added to water Na + H2O → Na+ + OH- + H2 • Addition Reactions - Simple synthesis sodium + chlorine 2 Na + Cl2 * → 2NaCl - Nonmetallic oxide and water CO2 + H2O→ H2CO3 - Metallic oxides in water MgO + SO2 → MgSO3 - Metallic oxides and nonmetallic oxides Na2O + CO2 → Na2CO3 • Combustion of hydrocarbons - Hexane is burned in air 2 C6 H14 +19 O2 → 12 CO2 + 14 H2O • Single replacement (net ionic) • Double replacement (net ionic) * Teach molecular first and net ionic after solutions. Nomenclature (binary ionic & covalent) Writing and balancing equations single, double, synthesis, decomposition, combustion of hydrocarbons Anhydrides taught with synthesis and decomposition reactions Calculation of oxidation numbers Oxidation, reduction, oxidizing agents, and reducing agents. Net ionic equations for single and double replacement (taught with solutions) Solubility rules The first two weeks start with memorization quizzes - solubility rules - strong acids/strong bases - diatomic molecules *Nomenclature should be reviewed as a summer assignment. Whether this will remain the case is not known, but if it does, a default “fair” question about such a (redox) reaction might have to do with a change in oxidation numbers which can be determined without having any idea what the reaction behavior is like. Below is a simplified set of rules which should look familiar. 1. the oxidation number of an element is 0 2. the oxidation number of monoatomic ion is equal to its charge 3. in compounds: the oxidation number of hydrogen is +1 (except in metal hydrides where it is -1) the oxidation number of oxygen is -2 (except in peroxides where it is -1) the oxidation number of alkali metals is +1 the oxidation number of alkaline earth metals is +2 the oxidation number of terminal halogens is -1 4. the sum of all the oxidation numbers in a molecule or ion is equal to the charge Students are quizzed every week starting in the following order: Anhydrides (2) weeks Additions Reactions & Decomposition (2) weeks Single & Double (ppt) (2 weeks) Redox (2 weeks) Combustion (2weeks) Complex Ions (2 weeks) *I teach in this order because I will have covered the topics in pre-AP or AP. Over the past 10 years the number of truly difficult non-trival reactions has dwindled. There have only been 2 and those were in years when you could easily have avoided them by choosing other options. Now that there are no options and reactions must be balanced it is questionable whether these kinds of reactions will ever appear again. It is clear that monoatomic ion redox will continue to be considered fair game. Here is an example from the 2007 exam: “A solution containing silver(I) ion (an oxidizing agent) is mixed with a solution containing iron(II) ion (a reducing agent)” Such reactions can be completed by following simple rules. The non-trivial sort require knowledge of common oxidizer/reducer pairs or at the very least some common-sense elimination of unlikely products followed by inspired guessing. Memorizing the "common" pairs may help but you will probably get farther by trying to reason through the process since there is no guarantee that the pairs you memorize will be used on the exam. Some essential principles to keep in mind: • elements in their highest positive oxidation state (same as group #, whether A or B) can ONLY be reduced • elements in their lowest oxidation state (0 for metals, negative for non-metals, corresponding to distance from noble gases) can ONLY be oxidized • intermediate oxidation states can go either way!!! • if the mixture is acidic, H+ should be included as a reactant; water is one product • if the mixture is basic, OH- should be included as a reactant; water is one product • occasionally the acid anion or base cation may precipitate with a product ion ***The last three items on the list may be irrelevant if truly complex non-trivial processes simply die a quiet death on future exams. oxidizers [remember, oxidizers will become reduced] MnO4- (in acid) → Mn2+ MnO4- (in neutral or basic) → MnO2 MnO2 (in acid) → Mn2+ Cr2O72- (in acid) → Cr3+ HNO3 (conc.) → NO2 HNO3 (dilute) → NO H2SO4 (hot, concentrated) → SO2 [if not hot and conc., this acts like HCl or other normal acids] metal cations → lower charge cations or (rarely) free metals free halogens → halide ions reducers [remember, reducers will become oxidized] halide ions → free halogens free metals → metal cations SO32- (or SO2) → SO42NO2- → NO3free halogens (dil. basic) → hypohalite ions [like XO-] free halogens (conc. basic) → halate ions [like XO3-] metal cations → higher charge cations Below are some reactions from past tests. 1. a solution containing tin(II) ions is added to acidified potassium dichromate solution --there is a color change during this reaction; which atom is most likely responsible? explain 2. powdered iron is added to a solution of iron(III) sulfate --which species are spectators? 3. solutions of tin(II) chloride and iron(III) chloride are mixed --both the reactant and product mixtures are colored; which ions account for the colors? 1. 3 Sn2+ + 14 H+ + Cr2O72- → 3 Sn4+ + 2 Cr3+ + 7 H2O --the chromium changes in oxidation state and is a transition metal; most transition metal compounds are colored and the color changes with oxidation state 2. Fe + 2 Fe3+ → 3 Fe2+ --the sulfate ion is a spectator 3. Sn2+ + 2 Fe3+ → Sn4+ + 2 Fe2+ --since iron is a transition metal it is likely that the colors come from Fe2+ and Fe3 OK! How much time do I spend on non-trivial redox? That depends on how much time you have. If on a modified block or period schedule, I suggest you spend very little time. It is worth the gamble with the new format. There are other topics that are much more important. The morning I introduce complex ions to my AP chem students. Reactions of coordination compounds and ions are not covered in depth on the exam but you will sometimes see them in the reaction-writing section and they are easy enough to complete with a few basic principles in mind. Most can be recognized by the choice of reactants: generally a transition metal ion or compound (also occasionally the amphoteric species from Group 3A such as Al) and a source of ligands. The most common ligands involved in questions are ammonia, the hydroxide ion, and the cyanide ion. Key to recognizing such is often the word "excess", indicating that enough of the complexation agent has been added to eliminate the possibility of precipitation of lesser-coordinated species. Occasionally this word will not appear and instead “concentrated” is used to descibe the added complexing agent (in that case usually an acid or base) One of the hurdles to get over is some knowledge of the likely coordination numbers for metal ions. Unfortunately there is no simple way to remember all of them. Some you may recognize from work done in the lab or something you read. In a pinch, it may be helpful to know that often the coordination number is twice the cation charge. In any case, you will seldom lose points just because you used a coordination number of 4 instead of 6. Historically, reactions involving complex ions on the exam fall into three broad categories: 1. complexation of a soluble salt e.g. a concentrated solution of ammonia is added to a solution of copper(II) chloride 4 NH3 + Cu2+ → [Cu(NH3)4]2+ 2. complexation of an insoluble salt e.g. excess concentrated potassium hydroxide solution is added to a precipitate of zinc hydroxide 2 OH- + Zn(OH)2 → [Zn(OH)4] 23. destruction of a complex by acid/base neutralization e.g. dilute hydrochloric acid is added to a solution of diamminesilver nitrate 2 H+ + Cl- + [Ag(NH3)2] + → AgCl + 2 NH4+ Examples of actual reactions from past A.P. exams along with added questions. 1. excess dilute nitric acid is added to a solution of tetramminecadmium(II) ion --what is the coordination number of the complex ion? 4 H+ + [Cd(NH3)4] 2+ → Cd2+ + 4 NH4+ --the coordination number of [Cd(NH3)4] 2+ is 4 2. pellets of aluminum metal are added to a solution containing an excess of sodium hydroxide --which reactant acts as a Lewis acid? explain 2. Al + 4 OH- → [Al(OH)4] --the Al accepts electron pairs from the hydroxide ions and is thus a Lewis acid 3. an excess of ammonia gas is bubbled through a solution saturated with silver chloride --which reactant acts as a Lewis base? explain 2 NH3 + AgCl → [Ag(NH3)2] + + Cl--the NH3 donates electron pairs to the silver ion and is thus a Lewis base 4. a concentrated solution of ammonia is added to a suspension of zinc hydroxide --what visual change occurs in the reaction mixture? . 4 NH3 + Zn(OH)2 → [Zn(NH3)4] 2+ + 2 OH--the suspension starts out slightly cloudy and ends up clear 5. a solution of ammonium thiocyanate is added to a solution of iron(III) chloride --describe the color changes that occur during the reaction SCN- + Fe3+ → [FeSCN] 2+ (other species up to CN6 accepted) --the original solutions are nearly colorless while the product mixture is orange to blood red After teaching complex ions. Was this ever taught in my college chemistry courses, especially if I have a comprehensive degree? Do you remember the qualitative analysis of cations lab? (Chem I) Again, do not re-invent the wheel. Quizzes are on the NMSI site. NMSI has great a resource by Kristen Henry. Organic is not included in her handout. Chemical Equations Handbook by George R. Hague and Jane O. Smith All quizzes should be cumulative. Don’t forget to add at least 3 to 5 questions about the reactions: -oxidizing agents -reducing agents - color - what is oxidized -what is reduced - how to test for gases- what gas is produced - what solid is produced - what ion stay in solution - what is losing electrons -what is gaining electrons - stoichiometry (There are not a lot of old AP test with this format. The format changed In 2007). Test format: 3 equations One question per equation Students must balance equation including charges. 1 point for products, 2 points for reactants 1 point for balancing, and 1 point for answering question correctly. This section is included in part B of free response (40 minutes) Students should attack problems by identifying type of reaction in this order. Synthesis Decompositions Single Replacement Double (ppt) Complex Ions Non-Trivial Redox Example: 2006 Question 4 Clue students in on wording - solutions - solids - the must know reacting species for strong acids and bases - concentrated/dilute - catalysis - two ions ( positive and negative) precipitation or addition - two ions (positive) redox - the last resort…look at reduction potential chart. • Have students start a list of common mistakes and clues. They should keep them in their notebook and add to the list after the quizzes are returned. • Make the list of common mistakes and reminders as you grade the quiz •Labs and demonstration are an excellent way of imbedding reactions into the curriculum. •Students remember what they see rather than symbols and formulas on a sheet of paper. •Also important due to the format change in 2007. This could be a lab question also.