Classification Of Matter
advertisement

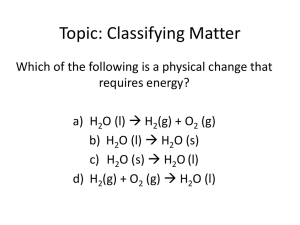
Classification Of Matter Integrated Science Dr. May Matter • Anything that has mass and occupies space is defined as matter • Matter may be invisible to the eye • All matter is composed of discrete, tiny particles called atoms Physical States Of Matter • All matter exists in one of three physical states: • Solid • Liquid • Gas Solid Matter • Definite shape and definite volume • Particles cohere rigidly to one another • Shape can be independent of the container • May be crystalline (salt, sugar, metal) or • May be amorphous (plastic, glass, gels) Liquid Matter • Definite volume but not a definite shape • Particles adhere firmly but not rigidly • Takes the shape of the storage container • Particles are in close contact with one another but are able to move freely Gaseous Matter • Indefinite volume and no fixed shape • Particles move independently of each other • Particles have gained enough energy to overcome the attractive forces that held them together as solids and liquids Substance • Matter with a definite, fixed composition is called a substance • Pure substances are either • Elements or • Compounds Homogeneous • Uniform in appearance • Same properties throughout • Water in each phase is homogeneous in composition Ice Is Solid H2O Heterogeneous • Matter having two or more physically distinct phases • Ice, water, and steam are each homogeneous • But, ice in water is heterogeneous and • Boiling water is heterogeneous Mixtures • A material containing two or more pure substances • Can be either • Homogeneous or • Heterogeneous Classification of Matter Matter Pure Substances (Homogeneous) Elements Compounds Mixture of two or more substances Solutions (Homogeneous Compositions) Heterogeneous Mixtures (Two or more phases) Physical Properties • Can be determined without altering the composition of the material • • • • Density Melting and boiling point Odor and color State of matter (solid, liquid, or gas) Chemical Property • Ability of a substance (H2) to form new substances (H2O) • By reacting with another substance (O2) 2 H2 + O2 2 H2O or • By decomposition H2CO3 H2O + CO2 Physical Change • Any change in a property of matter that does not result in a change in identity • • • • Chopping wood Freezing water Melting sugar Grinding coffee Chemical Change • Any change in which one or more substances are converted into different substances with different characteristic properties • Burning wood • Burning sugar • Adding sodium to water Indications Of Chemical Reactions • Evolution of heat and light Burning propane • Production of a gas Soda and vinegar • Formation of a precipitate Silver nitrate in salt water The End • This presentation was created for the benefit of our students by the Science Department at Howard High School of Technology • Please send suggestions and comments to rmay@nccvt.k12.de.us