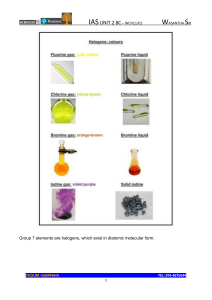
Group VII Chlorine to iodine Physical properties They are all non-polar molecules. Except fluorine, they are all slightly soluble in water. Their solubility decreases as we go down in the group. Halogens are more soluble in organic solvents. Their solubility increases as we descend in the group.* They are all coloured, their colours become darker as we go down in the group. Their melting/boiling points increase as we descend in the group. Chemical properties They are strong oxidising agents, as shown in their reactions with metals. Although their most common oxidation state is -1, they have various oxidation numbers in their compounds (except F). They occur naturally as compounds with metals (as metal halides), this is why they are called halogens (salt makers in ancient Greek). Reactions of the halogens Halogens will remove e- from any species that is less electronegative than them* For instance, halogens remove e- from metals and some metal ions (e.g. Fe2+). In other words, they increase their oxidation number. Halogens themselves gain e-, their oxidation number is decreased. e.g. 2K + Cl2 → 2KCl or 2Fe2+ + Br2 → 2FeBr3 * Halogens will also remove e- from halide ions as long as the halide comes from a halogen that is less reactive. e.g. 2KBr + Cl2 → 2KCl + Br2* Halogens will react with other non-metals, but they will form a polar covalent bond as their difference in electronegativity is equal to or smaller than 1. e.g. 2P + 3Cl2 → 2PCl3 or H2 + Br2 → 2HBr neither of these bonds is ionic as we saw before The speed of all of these reactions depends on the reactivity of the halogen. This is particularly well demonstrated in the reaction of halogens with hydrogen*. Disproportionation reactions of the halogens When one of the halogens is reacted with OHthe following reaction occurs: X2 + 2OH- → X- + XO- + H2O When performing this reaction in the lab we will see the colour of the halogen solution disappear. Rate of reaction will depend on the reactivity of the halogen and temperature.* On heating, XO- disproportionates to XO3- and X- : 3XO- → XO3- + 2XSo when an halogen reacts with hot OH-: 3X2 + 6OH- → XO3- + 5X- +3H2O Reactions of halide ions Reactions of halides with conc. H2SO4 Conc H2SO4 reacts with a chlorides to form hydrogen chloride. MgCl2 + 2H2SO4 → Mg(HSO4)2 + 2HCl In the case of bromides and iodides, the acid acts as an oxidising agent 2HBr + H2SO4 →Br2 + 2H2O + SO2* The iodides are oxidised more easily (and the acid reduced further) so in their reaction with H2SO4 the acid is reduced to H2S. H2SO4 + 8H+ + 8I- → H2S + 4H2O + 4I2* Testing for halide ions Silver nitrate is added to the solution to form silver halides. Then acid is used to prevent the formation of other silver salts as they dissolve in the presence of H+ ions.* The precipitate is then tested by adding NH3. The technique is based on the different solubilities in NH3 that these halides display* Hydrogen halides Reactions hydrogen halides with ammonia They react to form a salt* Reactions of hydrogen halides with water They react to form the corresponding acidic solution.*