whatsmattervocab

What ’ s the Matter
Game Definitions
Unit 2: Classification of Matter
Physical Science
Steinbrink
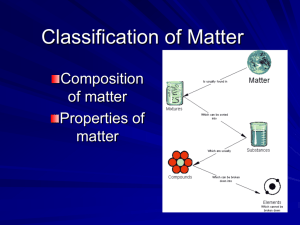
Matter
Anything that has mass and takes up space. Chemistry is the study of this!
Mixture
Most common type of matter, two or more substances having separate properties
Homogeneous Mixture
A mixture that is evenly mixed, the same throughout, can be separated by physical means
Heterogeneous
Mixture
Any type of mixture that is made up of different substances in different proportions, not evenly mixed.
Suspension
A Heterogeneous mixture in which one substance has groups of particles too heavy to remain mixed, eventually settling occurs.
Gel
A colloid in which a liquid is dispersed throughout a solid
Pure Substances
The type of matter chemists are most interested in, having identifiable properties, made up of units that are all alike.
Colloid
A mixture that can look like a solution but the different substances are held up in suspension, not truly dissolved, will scatter a light beam.
Compound
Pure substance made up of two or more types of atoms in a specific ratio, cannot be separated by physical change, has a formula.
Elements
Simplest substance of all, atoms are all alike with the same number of protons, cannot be separated, has a symbol, found on periodic table.
Emulsion
A colloid in which two liquids are dispersed but not truly dissolved.
Foam
A colloid in which a gas is dispersed throughout a liquid or a solid.
Solution
Any homogeneous mixture, particles of a solute are completely mixed between particles of a solvent.
Alloy
Solution of two or more metals with unique properties but not chemically bonded into a compound.