H - Chemistry Classes of Professor Alba
advertisement

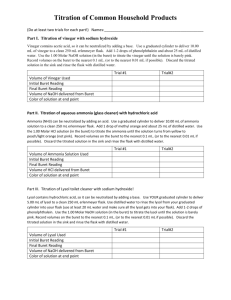
Exp 4C: How Much Acetic Acid is in Vinegar? Purpose – To use quantitative analysis and titrations to find the molarity of an acid or base. In this experiment, we shall determine the amount of acid in vinegar. Exp 4C: How Much Acetic Acid is in Vinegar? • Quantitative analysis – Determining the specific amount (molarity or concentration) of a particular substance in a sample • (qualitative analysis determines the presence w/o specifying the amount) – Examples • • • • • • Cholesterol levels in serum Iron in blood Lead in drinking water NO in air Drug tests for athletes Etc. Exp 4C: How Much Acetic Acid is in Vinegar? • Titration – Determining the amount of a substance by a reaction with another substance with known concentration – Detection of the chemical reaction by some kind of signal, e.g., color change of an indicator • Purpose of the Experiment – Determine the amount of acetic acid in vinegar by titration with sodium hydroxide CH3COOH + NaOH CH3COONa + H2O H+ + OH- H2O Neutralization Reaction – Indicator is phenolphthalein • Changes from colorless (acidic) to pink (basic) Exp 4C: How Much Acetic Acid is in Vinegar? • Analysis by Titration – Take a known volume of acetic acid – Add sodium hydroxide of a known concentration in small amounts CH3COOH + NaOH CH3COONa + H2O H+ + OH- H2O – As long as there is more acetic acid than sodium hydroxide, the solution reacts acidic • Indicator will be colorless Exp 4C: How Much Acetic Acid is in Vinegar? • Analysis by Titration (cont’d) – When acetic acid initially in solution equals sodium hydroxide added, you have reached the equivalence point (neutral solution) – When more sodium hydroxide than acetic acid is present, the solution turns basic • Indicator will become pink • When the solution becomes just pink with one drop of NaOH, you have reached the “endpoint” of the titration An Acid-Base Titration Addition of base until all acid is neutralized Base Indicator Acid Start of titration Excess of acid Point of neutralization Slight excess of base An Acid-Base Titration Point of neutralization H+(aq) + OH-(aq)] H2O(l) Point of neutralization (“Equivalence point”) All moles of H+ ions present in the original volume of acid have reacted with an equivalent amount of moles of OH- ions form the base added moles of H+ (originally in flask) = moles of OH- (added from buret) Exp 4C: How Much Acetic Acid is in Vinegar? • Analysis by Titration (cont’d) Equivalence Point CH3COOH + NaOH CH3COONa + H2O H+ + OH- H2O – Every single ion of H+ from acetic acid has reacted with an OH- ion from sodium hydroxide to form H2O • • • • Moles of acid = moles of base Moles of H+ = moles of OHMoles of acid = concentrationacid * volumeacid = mol/L * L Moles of base = concentrationbase * volumebase = mol/L * L – Calculation of acid-base concentrations for a molar ratio of 1:1 • Macid * Vacid = Mbase * Vbase if you know the volume of acid, the volume of base in a titration and the concentration of one of the components, you can calculate the concentration of the other Exp 4C: How Much Acetic Acid is in Vinegar? Experimental:Preparation (Different than in lab manual!!! Take notes) • Get 50 mL buret and 10 mL pipet • Measure about 30 mL of vinegar in one beaker and ~ 85 mL of NaOH solution, 0.20 M in another beaker • Place buret in clamp and add about 5 mL of NaOH solution using a funnel • Clean buret by carefully rotating and pouring out the 5mL NaOH solution into a waste beaker • Place buret back in buret clamp Exp 4C: How Much Acetic Acid is in Vinegar? Experimental: Trial Titration aka Rapid Titration (WHY?) • • • • Put 5.0 mL of vinegar in a clean 125 mL Erlenmeyer flask Add ~ 20 mL dH2O Add 2 drops of phenolphthalein Fill the buret with 50-mL of NaOH 1) Record the molarity of NaOH 2) Use a funnel to fill the buret, not over the 0.0 line 3) Record the initial volume (SIG FIGS!!!!) • • • • • • Put the tip of the buret in the Erlenmeyer, and a piece of white paper underneath the flask Add the NaOH solution in 1-mL increments to the acetic acid while swirling Note the color after swirling When you get close to the equivalence point, the solution will briefly turn pink and then colorless again Add smaller amounts until the solution stays pink for about 30 seconds RECORD VOLUME OF NaOH ADDED (final reading – initial reading) Exp 4C: How Much Acetic Acid is in Vinegar? Experimental: Exact Titration • • • • Put 5.0 mL of vinegar in a clean 125 mL Erlenmeyer flask Add ~ 20 mL dH2O Add 2 drops of phenolphthalein Fill the buret with 50-mL of NaOH 1) Record the molarity of NaOH 2) Use a funnel to fill the buret, not over the top line 3) Record the exact initial volume • • • • • Put the tip of the buret in the Erlenmeyer, and a piece of white filter paper underneath the flask Subtract about 1-2 mL from the volume determined in the trial titration Rapidly add the remainder to the flask Rinse the wall of the flask with dH2O (Why?) Continue adding NaOH drop-by-drop until the solution has a barely visible pink color that does not go away upon swirling Exp 4C: How Much Acetic Acid is in Vinegar? Experimental: Exact Titration (continued) • Record the volume of the buret • The volume added is Vfinal - Vinitial • Repeat the procedure • Calculate the molarity for each procedure using the formula for the titration. – • The difference must be not more than 2%. Calculate the molarity of the vinegar For next week and after: • Due March 26: 1. Results and post-lab for Exp 4C 2. No Pre-lab due • April 2: Long Quiz (See Study Guide given in class, to be posted): Exp. 1A, 3A, 3B, 4A, 4B a. Know the purpose and general techniques for each b. Be able to interpret results c. Understand and explain safety precautions d. Study the pre- and post-lab questions e. Know how to do all calculations