MonicaSchneider-Chemical analysis of common substances
advertisement

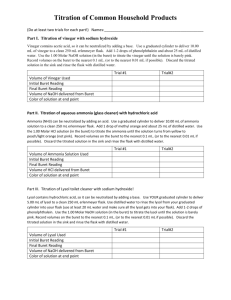
Lesson Plan Format Sinclair STEM Grant Teacher Instructions/Lesson Plan Template TITLE OF LESSON/MODULE Chemical analysis of common substances AUTHOR(S) AND SCHOOL AFFILIATION(S) Monica Schneider, Mason High School OVERVIEW Students will begin with basic lab concepts in a short lab experience. They will use those ideas to develop an experiment to analyze common substances just as they do in the Heinz Portion Quality Analysis Lab. TYPE/LEVEL OF INQUIRY 1= Open Inquiry 3= Guided Inquiry Initial lab is a 3. Analysis of common substances is a 1. GRADE LEVEL/COURSE Grade: 10-12, Chemistry ANTICIPATED LENGTH OF LESSON/MODULE (MINUTES) 2-3 class periods of 50-70 minutes. (150-200 minutes total) PREREQUISITE KNOWLEDGE Students need to have some understanding of pH, acids and bases and solubility of salts. STATE/NATIONAL STANDARDS Science Include the name of the Course (from the HS Syllabi) Include the Section of the Course Content that is included in the lesson. Highlight the subcategory, if appropriate. Cut and Paste the statements from the Content Elaboration that describe the content to be taught in the lesson/module. o Example – for a lesson that investigates the differences between meiosis and mitosis Course: Chemistry Course Content: Interactions of Matter Chemical reations Content Elaboration: Acids and Bases Acids often result when hydrogen is covalently bonded to an electronegative element and is easily dissociated from the rest of the molecule to bind with water to form a hydronium ion. The acidity of an aqueous solution can be expressed as pH, where pH can be calculated from the concentration of the hydronium ion. Bases are likely to dissociate in water to form a hydroxide ion. Acids can react with bases to form a salt and water. Such neutralization reactions can be studied quantitatively by performing titration experiments. Structure and Properties of Matter Intermolecular chemical bonding o Implications for properties of substances Solubility Content Elaboration: Substances will have a greater solubility when dissolving in a solvent with similar intermolecular forces. If the substances have different intermolecular forces, they are more likely to interact with themselves than the other substance and remain separated from each other. In order for an ionic substance to dissolve in water, the attractive forces between the ions must be overcome by the dipole-dipole interactions with the water. Science Inquiry and Application: During the years of grades 9-12, all students must use the following scientific processes with appropriate laboratory safety techniques to construct their knowledge and understanding in all science content areas: Identify questions and concepts that guide scientific investigations Design and conduct scientific investigations Use technology and mathematics to improve investigations and communications Formulate and revise explanations and models using logic and evidence (critical thinking) Recognize and analyze explanations and models Communicate and support a scientific argument MATERIALS (NEEDED PER LAB GROUP PER CLASS) 2 x 125 mL Erlenmeyer flasks 100 or 150 mL beaker vinegar distilled water 50 mL buret 6 x 250 mL beaker phenolphthalein buret stand and holder funnel standardized NaOH (aq) Analytical balance Bunsen burner 3 funnels plastic wash bottle 10-mL graduated cylinder 100-mL graduated cylinder ring stand iron ring wire gauze 3 stirring rods 3 rubber policemen 3 pieces of filter paper 3 watch glasses weighing paper INSTRUCTIONAL PLAN (INCLUDE STUDENT SHEETS SEPARATE FROM THIS DOCUMENT.) Write a detailed, step-by-step description of how to replicate the lesson and achieve the lesson plan objectives. This is usually intended for the teacher and provides suggestions on how to proceed with implementation of the lesson plan. It also focuses on what the teacher should have students do during the lesson. This section is divided into several components: an introduction, a main activity, and post-activity discussion. A. INTRODUCTION Teacher should have already completed some information about acids and bases and the calculations needed during titrations. Depending on the level of understanding, more or less of this information can be reviewed or discussed. Basic lab techniques such as folding the filter paper, buret set-up etc. should be discussed. Correct handling of dangerous chemicals should be discussed. B. ACTIVITY Students should receive the following hand out. This hand out begins with a basic lab for each test. Then it allows the students to take that information and expand on it at their own pace. Heinz Quality Analysis Lab At the lab in the Heinz Portion Control plant in Mason, Ohio, they must test every batch of sauce, salad dressing and jelly that is made. Many tests are performed for each substance that goes through the lab. The tests include: pH of the sauce or solution, titration of the sauce or solution and amount of sodium chloride in the sauce or solution. In this lesson, you will first practice the techniques with common laboratory substances. Ultimately, your job is to test the sauces from local restaurants just as they do in the QA lab. Use of the pH meter: 1. Connect the pH Sensor to the computer interface. 2. Raise the pH Sensor from the sensor storage solution and set the solution aside. Use a wash bottle filled with distilled water to thoroughly rinse the tip of the sensor as demonstrated by your instructor. Catch the rinse water in a 250 mL beaker. 3. Get one of the solutions in the small container supplied by your sensor. Raise the solution to the pH Sensor and swirl the solution about the sensor. When the pH reading stabilizes, record the pH value in your data table. 4. Prepare the pH Sensor for reuse. a. Rinse it with distilled water from a wash bottle. b. Place the sensor into the sensor soaking solution and swirl the solution about the sensor briefly. c. Rinse with distilled water again. 5. Determine the pH of the other solutions. You must clean the sensor between tests. When you are done, rinse the tip of the sensor with distilled water and return it to the sensor soaking solution. Titration using pH meter: Introduction Vinegar is a common household item that is found in a number of products from salad dressing to cleaners. Vinegar is a solution of acetic acid (CH3COOH or HC2H3O2) in water. The amount of acetic acid is usually 5% by mass in the vinegar solution. In this experiment, you will determine the mass percent of acetic acid in vinegar by titration. Titration is a common method used by chemists to find the concentration of a substance in a solution. Titration involves two key components: the titrant and the analyte. The titrant is a solution of known concentration which is used to find the concentration of the analyte, a solution of unknown concentration. Acid-base titrations are the most common type of titration. If the analyte is an acid, then the titrant is a base. The titrant would be added to the analyte until all of the acid is neutralized -- this is known as the equivalence point or end-point. At the equivalence point, the number of moles of acid (H+) is equal to the number of moles of the base (OH−)(based on stoichiometry). By carefully measuring the amount of titrant used, you can determine the number of moles of acid present. The easiest way to determine the equivalence point of the reaction taking place is to use a visual indicator. Visual indicators change color at different pH. For this titration the indicator is phenolphthalein, which changes from colorless (pH < 8) to pink (pH > 8). The analyte for this experiment is the acetic acid in vinegar with pH less than 7. Our titrant will be sodium hydroxide, a base. When enough titrant, NaOH, is added to neutralize the acetic acid, the solution will change from colorless to just barely pink in color. NaOH(aq) + HC2H3O2(aq) → H2O(l) + NaC2H3O2(aq) It is important that you measure all volumes precisely. You must know the exact amount (to 0.01 mL) of NaOH(aq) required to react with the HC2H3O2 as well as the amount of HC2H3O2 you began with. The reaction of NaOH with HC2H3O2 is 1:1 stoichiometrically. Since you will measure the volume of NaOH(aq) added and be given the molar concentration of the NaOH, you can find the moles of NaOH. The moles of NaOH will be equal to the moles of HC2H3O2 in the solution. To get percent by mass of HC2H3O2 in vinegar, you will need to know the molar mass of the HC2H3O2 and the mass of the vinegar sample. Laboratory Activity Materials: 2 x 125 mL Erlenmeyer flasks 100 or 150 mL beaker vinegar distilled water 50 mL buret 200 or 250 mL beaker phenolphthalein buret stand and holder funnel standardized NaOH (aq) Procedure 1. Rinse a 50 mL buret two or three times with deionized water. Be sure to let the water run out of the tip. 2. Rinse and dry the 250 mL beaker. Pour ~100 mL of NaOH into the beaker. 3. Obtain and clean a funnel then add about 5 mL of the NaOH(aq) to the buret. Rinse the buret with the NaOH(aq) making sure to rotate the buret so that the sides are coated with the solution. Let some of the NaOH(aq) out of the tip and pour the rest down the sink. Repeat with another 5 mL of NaOH(aq). 4. Fill the buret with the NaOH solution until the volume reads a little above the 0.00 mL line. Drain the buret into a waste beaker until the buret reads 0.00 mL. The tip of the buret should be completely filled with solution – any air bubbles present will interfere with your measurements. If there is an air bubble in the tip, continue draining until the bubble comes out then refill the buret to 0.00 mL. 5. Obtain two Erlenmeyer flasks and rinse them well with deionized water ( you do not need to dry them). The vinegar dispenser in lab will dispense 3.00 mL of vinegar per pump; use this to add 3.00 mL of vinegar to each flask. Add about 25 mL of deionized water and one drop (only 1 drop) of phenolphthalein to each flask. 6. Titrate the vinegar solution by carefully adding NaOH(aq) from the buret into the Erlenmeyer flask containing the vinegar. Gently swirl the flask constantly to mix. Stop the titration when a faint pink color appears. Record the volume added to 0.01 mL. This is the equivalence point. If the color is hot pink, start over with a new sample of vinegar. 7. Repeat this titration until that every member of your group has done two titrations. Disposal Pour the unused NaOH(aq) from the buret back into the bottle. Pour the remaining solutions down the drain. Clean by rinsing well with deionized water two or three times (especially the tip!) and return the buret. Calculations The percent by mass of acetic acid present in a sample of vinegar is determined by first finding the amount of acetic acid present in the sample titrated. The moles of acetic acid are equal to the moles of NaOH used in the titration as the reaction follows a 1:1 stoichiometry. The moles of acetic acid are then converted to grams using the molar mass. Calculation of NaCl in a substance 1. Add about .4 g of the unknown sample on weighing paper. Transfer the sample to a clean 250-mL beaker. Label the beaker #1. 2. Add approximately 150 mL of distilled water and 1 mL of 6 M HNO3 to the beaker. 3. Repeat steps 1 and 2 twice, with subsequent beakers labeled #2 and #3. 4. Using a different glass rod for each solution, stir the contents of the beakers until the sample had dissolved in each. Leave the stirring rods in the beakers after stirring. 5. While stirring each solution, add approximately 20 mL of 0.5 M AgNO3 solution. Place a watch glass over each beaker. Gently warm each solution with a Bunsen burner and keep warm for 10 minutes. Do not allow solutions to come to boil. 6. Label with the beaker number and weigh three filter papers. Record the masses. Fold the paper and place into the funnel. Wet each filter paper with distilled water to hold it in place in the funnel. 7. Transfer the precipitate and the warm water from each beaker into each funnel. Use a rubber policeman and a wash bottle to obtain the last traces of precipitate from each beaker. Keep the level of solution in each filter funnel below the top of the filter paper. 8. After all of the precipitate had been transferred from beaker to funnel, pour approximately 5 mL of distilled water through the filter three times. Pour 5 mL of acetone was poured through the filter. 9. Remove the filter paper from each funnel and place on a watch glass. Store in hood overnight. 10. After the precipitate on the filter paper had been thoroughly dried overnight, obtain the mass of each filter paper. 11. Write the balanced chemical equation to determine what the precipitate is. Use your solubility rules to determine what the precipitate could be. 12. Use stoichiometric calculations to determine the amount of sodium chloride that was present in the original substances. The Challenge Heinz uses these techniques to determine the pH of the substance, the concentration of hydronium ions with the substance in an aqueous solution and the amount of salt (NaCl) in the substance. You are to obtain two different types of restaurant packets and test for the pH of the sauce, the concentration of acid by titration and the amount of salt. Pace yourself and remember that it takes time to dry the substances in the sodium chloride test! Extension 1. Find another test that can be completed in the lab. Write up the complete procedure and carry out the test for extra credit! 2. You want to build a first-class quality analysis lab facility. What scientific equipment would you need to outfit this facility? Remember to include both technological equipment, glassware and chemicals to do the quality analysis over and over in a full-time (24 hours a day) factory. C. POST ACTIVITY DISCUSSION Students will complete the calculations and turn in their results. EXTENSIONS 1. Find another test that can be completed in the lab. Write up the complete procedure and carry out the test. Make sure you include the information that you found on the your data table with your other results. 2. You want to build a first-class quality analysis lab facility. What scientific equipment would you need to outfit this facility? Remember to include both technological equipment, glassware and chemicals to do the quality analysis over and over in a full-time (24 hours a day) factory. ASSESSMENT Final calculations should be graded for correctness. The teacher should test the substances to gather an “accepted value”. The teacher can or does not have to grade the students on how close they came to the accepted value. REFERENCES “Gravimetric Analysis of a Chloride Salt”. Yaksic.com. 14 June 2013. <http://www.yaksic.com/gacs8.html>. Holmquist, Dan, et al. Chemistry with Vernier. Beverton: Vernier, 2007. Print. “The Titration of Vinegar.” nvcc.edu. 14 June 2013. <http://www.nvcc.edu/alexandria/stb/chm/111/111.07VinegarTitrationSummer2013.pdf>. SAFETY CONSIDERATIONS NaOH and HNO3 are hazardous chemicals. Handle according to proper MSDS procedures. AgNO3 will stain hands and clothing brown. Handle with care. Heating substances over a Bunsen burner can be the cause of accidents. Always handle hot materials with care.