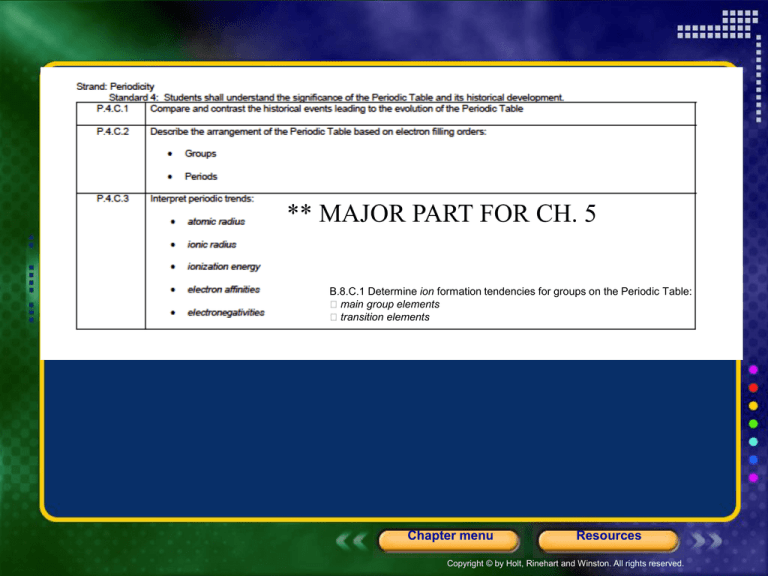
** MAJOR PART FOR CH. 5
B.8.C.1 Determine ion formation tendencies for groups on the Periodic Table:
main group elements
transition elements
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
OPENER #10 - FRI - Nov. 8, 2013 (B day) AND
Mon - Nov. 11, 2013 (A Day)
Pick up computer and log-in as you start opener.
1. Write the noble gas e- configuration for Phosphorus, P.
2. What is the greatest outer energy sublevel for Chlorine, Cl?
3. Draw the orbital notation for Vanadium, V (atomic #23).
I NEED ALL LABS TODAY!
CW: Notes 5.1-5.2
CW: Periodic Table Puzzle Puzzle Activity
CW/HW: Chapter 4 review questions pg. 124-126 #1, 311, 13-41, (1-41 except #2 & 12) due by Tuesday (B day)
and Wed (A Day).
TEST - TUESDAY ch. 4 - B Day - WED. A Day - Make an A!
Retake Element-Symbol Test before Thanksgiving!
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Energy Level Order - lowest to highest
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s,
etc.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
ASSIGNMENTS - FRI and MON 11-8 and 11-11-13
Homework:
Chapter 4 review questions pg. 124-126
#1, 3-11, 13-41, (1-41 except #2 & 12)
due by Tuesday (B day) and Wed (A
Day).
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
The Periodic Law
Table of Contents
Section 1 History of the Periodic Table
Section 2 Electron Configuration and the Periodic Table
Section 3 Electron Configuration and Periodic
Properties
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 1 History of the Periodic
Table
Lesson Starter
Share what you have learned previously about the
periodic table.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 1 History of the Periodic
Table
Objectives
• Explain the roles of Mendeleev and Moseley in the
development of the periodic table.
• Describe the modern periodic table.
• Explain how the periodic law can be used to predict
the physical and chemical properties of elements.
• Describe how the elements belonging to a group
of the periodic table are interrelated in terms of
atomic number.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
5.1
Organizing the Elements
In a self-service store, the
products are grouped
according to similar
characteristics. With a logical
classification system, finding
and comparing products is
easy. You will learn how
elements are arranged in the
periodic table and what that
arrangement reveals about
the elements.
Slide
of 28
8
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Searching For an Organizing
Principle
Searching For an Organizing Principle
How did chemists begin to organize the
known elements?
Slide
of 28
9
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Searching For an Organizing
Principle
Chemists used the properties of elements
to sort them into groups.
Slide
of 28
10
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Searching For an Organizing
Principle
Chlorine, bromine, and iodine have very
similar chemical properties.
Slide
of 28
11
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Mendeleev’s Periodic Table
Mendeleev’s Periodic Table
How did Mendeleev organize his
periodic table?
Slide
of 28
12
© Copyright Pearson Prentice Hall
End Show
Chapter 5
Section 1 History of the Periodic
Table
Mendeleev and Chemical Periodicity
• Mendeleev noticed that when the elements were
arranged in order of increasing atomic mass, certain
similarities in their chemical properties appeared at
regular intervals.
• Repeating patterns are referred to as periodic.
• Mendeleev created a table in which elements with
similar properties were grouped together—a periodic
table of the elements.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
5.1
Organizing the Elements
>
Mendeleev’s Periodic Table
An Early Version of Mendeleev’s Periodic Table
Slide
of 28
14
© Copyright Pearson Prentice Hall
End Show
Chapter 5
Section 1 History of the Periodic
Table
Mendeleev and Chemical Periodicity,
continued
• After Mendeleev placed all the known elements in his
periodic table, several empty spaces were left.
• In 1871 Mendeleev predicted the existence and
properties of elements that would fill three of the
spaces.
• By 1886, all three of these elements had
been discovered.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 1 History of the Periodic
Table
Properties of Some Elements Predicted By Mendeleev
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 1 History of the Periodic
Table
Moseley and the Periodic Law
• In 1911, the English scientist Henry Moseley
discovered that the elements fit into patterns better
when they were arranged according to atomic number,
rather than atomic weight.
• The Periodic Law states that the physical and
chemical properties of the elements are periodic
functions of their atomic numbers.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
6.1
Organizing the Elements
>
The Periodic Law
The periodic law: When elements are arranged
in order of increasing atomic number, there is a
periodic repetition of their physical and chemical
properties.
• The properties of the elements within a period
change as you move across a period from left
to right.
• The pattern of properties within a period
repeats as you move from one period to the
next.
Slide
of 28
18
© Copyright Pearson Prentice Hall
End Show
Chapter 5
Section 1 History of the Periodic
Table
Periodicity of Atomic Numbers
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 1 History of the Periodic
Table
The Modern Periodic Table
• The Periodic Table is an arrangement of the elements
in order of their atomic numbers so that elements with
similar properties fall in the same column, or group.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Visual Concepts
Periodic Table Overview
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_003
Visual Concept
036809x/student/ch05/sec01/v
c00/hc605_01_v00fs.htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Metals, Nonmetals, and Metalloids
What are three broad classes of
elements?
Slide
of 28
22
© Copyright Pearson Prentice Hall
End Show
6.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Three classes of elements are metals,
nonmetals, and metalloids.
Across a period, the properties of
elements become less metallic and more
nonmetallic.
Slide
of 28
23
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Metals, Metalloids, and Nonmetals in the Periodic Table
Slide
of 28
24
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Metals, Metalloids, and Nonmetals in the Periodic Table
Slide
of 28
25
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Metals, Metalloids, and Nonmetals in the Periodic Table
Slide
of 28
26
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Metals, Metalloids, and Nonmetals in the Periodic Table
Slide
of 28
27
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Metals
Metals are good conductors of heat and electric
current.
• 80% of elements are metals.
• Metals have a high luster, are ductile, and are
malleable.
Slide
of 28
28
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Uses of Iron, Copper, and Aluminum
Slide
of 28
29
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Uses of Iron, Copper, and Aluminum
Slide
of 28
30
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Uses of Iron, Copper, and Aluminum
Slide
of 28
31
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Nonmetals
In general, nonmetals are poor conductors of
heat and electric current.
• Most nonmetals are gases at room
temperature.
• A few nonmetals are solids, such as sulfur
and phosphorus.
• One nonmetal, bromine, is a dark-red liquid.
Slide
of 28
32
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
Metalloids
A metalloid generally has properties that are
similar to those of metals and nonmetals.
The behavior of a metalloid can be controlled by
changing conditions.
Slide
of 28
33
© Copyright Pearson Prentice Hall
End Show
5.1
Organizing the Elements
>
Metals, Nonmetals, and Metalloids
If a small amount of boron is mixed with silicon,
the mixture is a good conductor of electric
current. Silicon can be cut into wafers, and used
to make computer chips.
Slide
of 28
34
© Copyright Pearson Prentice Hall
End Show
Chapter 5
Section 1 History of the Periodic
Table
The Modern Periodic Table
• Ramsay discovered group 18 or 8A which are known as
the noble gases. These were more difficult to discover
because they are mostly nonreactive.
The lanthanides are the 14 elements from atomic
numbers 58 (cerium, Ce) to 71 (lutetium, Lu).
The actinides are the 14 elements from atomic numbers
90 (thorium, Th) to 103 (lawrencium, Lr).
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Section Assessment
Assess students’ understanding of
the concepts in Section
5.1
Continue to:
-or-
Launch:
Section Quiz
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
5.1 Section Quiz
1. The modern periodic table has elements
arranged in order of
a. colors.
b. melting and boiling points.
c. increasing atomic mass.
d. increasing atomic number.
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
5.1 Section Quiz
2. Mendeleev arranged the elements in his
periodic table in order of increasing
a. atomic number.
b. number of protons.
c. number of electrons.
d. atomic mass
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
5.1 Section Quiz
3. Which one of the following is NOT a general
property of metals?
a. ductility
b. malleability
c. having a high luster
d. poor conductor of heat and electricity
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
Online Self-Check Quiz
Complete the online Quiz and record answers.
Ask if you have any questions about your
answers.
click here for online Quiz 5.1
(8 questions)
You must be in the “Play mode” for the
slideshow for hyperlink to work.
Slide
of 25
© Copyright Pearson Prentice Hall
End Show
VIDEOS FOR ADDITIONAL INSTRUCTION
Additional Videos for Section 5.1: History of the Periodic Table
•Periodic Table Overview (4:31)
•Noble Gases (2:43)
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Lesson Starter
• Name as many properties shared by elements of the
same group in the periodic table as possible.
• Describe what you already know about an element
just by looking at its position in the periodic table.
• Identify any noticeable trends.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Objectives
• Explain the relationship between electrons in
sublevels and the length of each period of the
periodic table.
• Locate and name the four blocks of the periodic
table. Explain the reasons for these names.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Objectives, continued
• Discuss the relationship between group
configurations and group numbers.
• Describe the locations in the periodic table and the
general properties of the alkali metals, the alkalineearth metals, the halogens, and the noble gases.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table
• Elements are also organized horizontally in rows,
or periods. The period of an element can be determined by the
element’s highest energy level.
• Ex. As has the following noble gas electron configuration
• [Ar] 3d10 4s2 4p3 ➯ highest energy level is 4th energy level.
• Elements are arranged vertically in the periodic table in
groups (also called families) that share similar chemical
properties.
• The length of each period is determined by the number of
electrons that can occupy the sublevels being filled in that
period.
• The periodic table is divided into four blocks, the s, p,
d, and f blocks. The name of each block is determined
by the electron sublevel being filled in that block.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Visual Concepts
Relating Period Length and Sublevels Filled
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_003036809x/
Visual Concept
student/ch05/sec02/vc00/hc605_02_v0
0fs.htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Visual Concepts
Blocks of the Periodic Table Based on
Sublevel
Click below to watch the Visual Concept.
Visual Concept
http://my.hrw.com/sh/hc6_003036809x/stude
nt/ch05/sec02/vc01/hc605_02_v01fs.htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
6.2
Classifying the Elements
>
Squares in the Periodic Table
Slide
of 28
49
© Copyright Pearson Prentice Hall
End Show
5.2
Classifying the Elements
>
Transition Elements
Blocks of Elements
Slide
of 28
50
© Copyright Pearson Prentice Hall
End Show
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
• The elements of Group 1 of the periodic table are
known as the alkali metals.
• lithium, sodium, potassium, rubidium, cesium, and francium
• In
their pure and
state,
allsoft
of the
alkali to
metals
have
a silvery
appearance
are
enough
cut with
a knife.
• Hydrogen has an electron configuration of 1s1, but
despite the ns1 configuration, it does not share the
same properties as the elements of Group 1.
• Hydrogen is a unique element.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
6.2
Classifying the Elements
>
Electron Configurations in Groups
In atoms of the Group 1A elements below, there
is only one electron in the highest occupied
energy level. SEE THE CONNECTION
between the periodic table & electron
configuration for Group 1 alkali metal elements.
(Note the “ns1” highest energy level
configuration..)
Slide
of 28
52
© Copyright Pearson Prentice Hall
End Show
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
• The elements of Group 2 of the periodic table are
called the alkaline-earth metals.
• beryllium, magnesium, calcium, strontium, barium, and radium
• Group 2 metals are less reactive than the alkali metals, but are
still too reactive to be found in nature in pure form.
• Like the Group 2 elements, helium has an ns2
group configuration. Yet it is part of Group 18.
• Because its highest occupied energy level is filled
by two electrons, helium possesses special
chemical stability.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Relationship Between Periodicity and
Electron Configurations
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem A
a. Without looking at the periodic table, identify the
group, period, and block in which the element that has
the electron configuration [Xe]6s2 is located.
b. Without looking at the periodic table, write the
electron configuration for the Group 1 element in the
third period. Is this element likely to be more reactive
or less reactive than the element described in (a)?
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem A Solution
a. The element is in Group 2, as indicated by the group
configuration of ns2.
It is in the sixth period, as indicated by the highest
principal quantum number in its configuration, 6.
The element is in the s block.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem A Solution, continued
b. In a third-period element, the highest occupied
energy level is the third main energy level, n = 3.
The 1s, 2s, and 2p sublevels are completely filled.
This element has the following configuration:
1s22s22p63s1 or [Ne]3s1
Because it is in Group 1, this element is likely to be
more reactive than the element described in (a),
which is in Group 2.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
TEXTBOOK
PRACTICE
PROBLEMS
Now practice pg. 143 #1, 2a, 2b, 2c
If you need more room, you may attach notebook paper.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
TEXTBOOK
PRACTICE
PROBLEMS
Now practice pg. 143 #1, 2a, 2b, 2c
If you need more room, you may attach notebook paper.
DID YOU GET THESE ANSWERS?
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Section 2 Electron Configuration and
the Periodic Table
Chapter 5
Periods and Blocks of the Periodic Table
• The d sublevel first appears when n = 3. The d-block
elements are found in Groups 3-12.
• The 3d sublevel is slightly higher in energy than the 4s
sublevel, so these are filled in the order 4s3d.
• The d-block elements are metals with typical metallic
properties and are often referred to as transition
elements.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table
• 3 unreactive metals that exists in
nature as free elements include
palladium, platinum, and gold.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem B
An element has the electron configuration [Kr]4d55s1.
Without looking at the periodic table, identify the
period, block, and group in which this element is
located. Then, consult the periodic table to identify this
element and the others in its group.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem B Solution
• The number of the highest occupied energy level is 5, so the
element is in the fifth period.
• There are five electrons in the d sublevel, which means that it is
incompletely filled. The d sublevel can hold 10 electrons.
Therefore, the element is in the d block.
• For d-block elements, the number of electrons in the ns sublevel
(1) plus the number of electrons in the (n 1)d sublevel (5) equals
the group number, 6.
• This Group 6 element is molybdenum. The others
in Group 6 are chromium, tungsten, and seaborgium.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Practice problems pg. 146 #1-2
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Practice problems pg. 146 #1-2
DID YOU GET THESE ANSWERS?
#2 can also be written as 5s2 4d10
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
• The p-block elements consist of all the elements of
Groups 13–18 except helium.
• The p-block elements together with the s-block
elements are called the main-group elements.
• The properties of elements of the p block vary greatly.
• At its right-hand end, the p block includes all of the nonmetals
except hydrogen and helium.
• All six of the metalloids are also in the p block.
• At the left-hand side and bottom of the block, there are eight
p-block metals.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table, continued
• The elements of Group 17 are known as the halogens.
• fluorine, chlorine, bromine, iodine, and astatine
• The halogens are the most reactive nonmetals because they only
need 1 more electron to have a filled energy level.
• They react vigorously with most metals to form examples of the
type of compound known as salts.
• The metalloids, or semiconducting elements, are
located between nonmetals and metals in the p block.
• The metals of the p block are generally harder and
denser than the s-block alkaline-earth metals, but softer
and less dense than the d-block metals.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem C
Without looking at the periodic table, write the outer
electron configuration for the Group 14 element in
the second period. Then, name the element, and
identify it as a metal, nonmetal, or metalloid.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table, continued
Sample Problem C Solution
• The group number is higher than 12, so the element
is in the p block.
• The total number of electrons in the highest
occupied s and p sublevels is therefore equal to the
group number minus 10 (14 10 = 4).
• Two electrons are in the s sublevel, so two electrons
must also be present in the 2p sublevel.
• The outer electron configuration is 2s22p2.
• The element is carbon, C, which is a nonmetal.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
TEXTBOOK
PRACTICE
PROBLEMS
Now practice pg. 148 #1-2
If you need more room, you may attach notebook paper.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
TEXTBOOK
PRACTICE
PROBLEMS
Now practice pg. 148 #1-2
If you need more room, you may attach notebook paper.
DID YOU GET THESE ANSWERS?
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table, continued
• In the periodic table, the f-block elements are wedged
between Groups 3 and 4 in the sixth and seventh
periods.
• Their position reflects the fact that they involve the filling of the 4f
sublevel.
• The first row of the f block, the lanthanides, are shiny
metals similar in reactivity to the Group 2 alkaline
metals.
• The second row of the f block, the actinides, are
between actinium and rutherfordium. The actinides are
all radioactive.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem D
Name the block and group in which each of the
following elements is located in the periodic table.
Then, use the periodic table to name each element.
Identify each element as a metal, nonmetal, or
metalloid. Finally, describe whether each element has
high reactivity or low reactivity.
a. [Xe]4f145d96s1
c. [Ne]3s23p6
b. [Ne]3s23p5
d. [Xe]4f66s2
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem D Solution a. [Xe]4f145d96s1
a. The 4f sublevel is filled with 14 electrons. The 5d sublevel is partially
filled with nine electrons. Therefore, this element is in the d block.
The element is the transition metal platinum, Pt, which is in Group 10
and has a low reactivity.
b. [Ne]3s23p5
b. The incompletely filled p sublevel shows that this element is in the
p block.
A total of seven electrons are in the ns and np sublevels, so this
element is in Group 17, the halogens.
The element is chlorine, Cl, and is highly reactive.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 5
Section 2 Electron Configuration and
the Periodic Table
Periods and Blocks of the Periodic Table,
continued
Sample Problem D Solution, c. [Ne]3s23p6
c. This element has a noble-gas configuration and thus is in Group
18 in the p block.
The element is argon, Ar, which is an unreactive nonmetal and a
noble gas.
d. [Xe]4f66s2
The incomplete 4f sublevel shows that the element is in the f
block and is a lanthanide.
Group numbers are not assigned to the f block.
The element is samarium, Sm. All of the lanthanides are
reactive metals.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
TEXTBOOK
PRACTICE
PROBLEMS
Now practice pg. 149 #1a and 1b
If you need more room, you may attach notebook paper.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
TEXTBOOK
Now practice pg. 149 #1a and 1b
PRACTICE
PROBLEMS
If you need more room, you may attach notebook paper.
DID YOU GET THESE ANSWERS?
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
6.2
Classifying the Elements
>
Transition Elements
Blocks of Elements
Slide
of 28
78
© Copyright Pearson Prentice Hall
End Show
Online Self-Check Quiz
Complete the online Quiz and record answers.
Ask if you have any questions about your
answers.
click here for online Quiz 5.2
(9 questions)
You must be in the “Play mode” for the
slideshow for hyperlink to work.
Slide
of 25
© Copyright Pearson Prentice Hall
End Show
ASSIGNMENTS - FRI and MON 11-8 and 11-11-13
Homework:
Chapter 4 review questions pg. 124-126
#1, 3-11, 13-41, (1-41 except #2 & 12)
due by Tuesday (B day) and Wed (A
Day).
TEST also on the same days.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
VIDEOS FOR ADDITIONAL INSTRUCTION
Additional Videos for
Section 5.2: Electron Configuration and the Periodic Table
•Electron Configuration (3:53)
•s-Block Elements (5:00)
•d-Block Elements - f-Block Elements (2:06)
•p-Block Elements (1:13)
•Halogens (2:57)
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
Section Assessment
Assess students’ understanding of
the concepts in Section
5.2.
Continue to:
-or-
Launch:
Section Quiz
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
5.2 Section Quiz
1. Which of the following information about
elements is usually NOT included in a
periodic table?
a.
color
b.
symbol
c.
atomic number
d.
atomic mass
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
5.2 Section Quiz
2. An alkali metal would have in the highest
occupied energy level
a.
an s2 electron.
b.
an s1 electron.
c.
p2 electrons.
d.
p6 electrons.
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
5.2 Section Quiz
3. Which one of the following is incorrectly
labeled?
a. Ne, noble gas
b. Cu, transition metal
c. Ga, transition metal
d. Cl, halogen
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
5.2 Section Quiz
4. Transition metals are characterized as being
different than representative elements
because they have electrons in which
suborbitals?
a.
p
b. d
c. s
d. f
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
END OF SLIDE SHOW SECTION 5.2
Working with a partner, complete the periodic
table puzzle clue activity.
Be prepared to take your test on Tuesday (B) or
Wednesday (A day) - Be sure you completed
the chapter review homework pg. 124-126
#1-41 except #2 and 12.
© Copyright Pearson Prentice Hall
Slide
of 28
End Show









