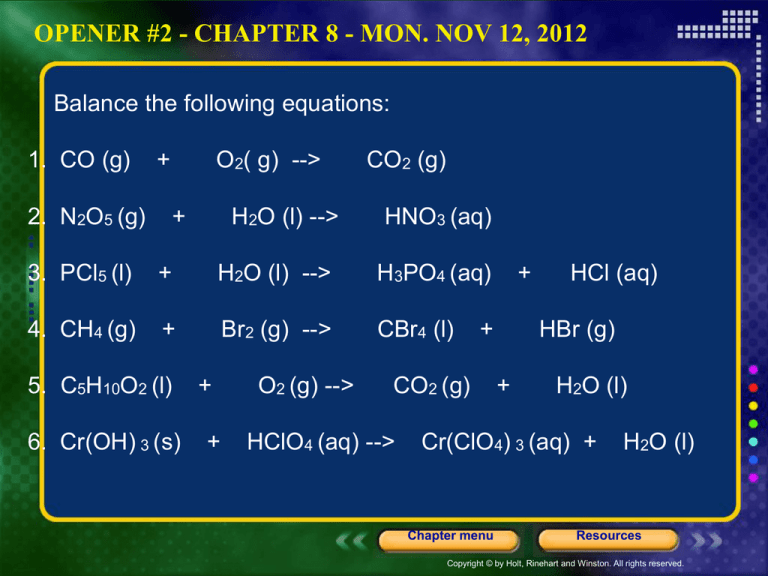
OPENER #2 - CHAPTER 8 - MON. NOV 12, 2012
Balance the following equations:
1. CO (g)
+
2. N2O5 (g)
O2( g) -->
+
H2O (l) -->
CO2 (g)
HNO3 (aq)
3. PCl5 (l)
+
H2O (l) -->
H3PO4 (aq)
4. CH4 (g)
+
Br2 (g) -->
CBr4 (l)
5. C5H10O2 (l)
6. Cr(OH) 3 (s)
+
+
O2 (g) -->
+
CO2 (g)
HClO4 (aq) -->
+
HCl (aq)
HBr (g)
+
H2O (l)
Cr(ClO4) 3 (aq) +
Chapter menu
H2O (l)
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
S.15.C.6 Identify the physical state for each substance in a reaction equation
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 8
Chemical Equations and Chemical
Reactions
Table of Contents
Section 3 Activity Series of the Elements
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 8
Section 3 Activity Series of the
Elements
Objectives
• Explain the significance of an activity series.
• Use an activity series to predict whether a given
reaction will occur and what the products will be.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 8
Section 3 Activity Series of the
Elements
• The ability of an element to react is referred to as the
element’s activity.
• The more readily an element reacts with other substances, the
greater its activity is.
• An activity series is a list of elements organized
according to the ease with which the elements
undergo certain chemical reactions.
• For
metals,togreater
activityions.
means a greater ease of loss of
electrons,
form positive
• For nonmetals, greater activity means a greater ease of gain
of electrons, to form negative ions.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 8
Section 3 Activity Series of the
Elements
• The order in which the elements are listed is usually
determined by single-displacement reactions.
• The
most-active element is placed at the top in the
series.
• It can replace each of the elements below it from a
compound in a single-displacement reaction.
• certain
Activity chemical
series arereactions
used to help
predict whether
will occur.
• Activity series are based on experiment.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
11.2
Types of Chemical Reactions
>
Classifying Reactions
The activity series of
metals lists metals in
order of decreasing
reactivity.
Slide
of 42
7
© Copyright Pearson Prentice Hall
End Show
Chapter 8
Section 3 Activity Series of the
Elements
Activity Series of the
Elements
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 8
Visual Concepts
Activity Series
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_00303680
Visual Concept
9x/student/ch08/sec03/vc00/hc608_0
3_v00fs.htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Practice Problems pg. 287 #1-3
Complete practice problems pg. 287 #1-3 in your notes.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
ANSWERS (for me)
Practice Problems pg. 287 #1-3
Complete practice problems pg. 287 #1-3 in your notes.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Section Review
8.3 p 287 #1-4
Complete 8.3 section review questions #1-4.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
ANSWERS (for me only)
Section Review
8.3 p 287 #1-4
Complete 8.3 section review questions #1-4.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Online Self-Check Quiz
Complete the online Quiz and record answers.
Ask if you have any questions about your
answers.
click here for online Quiz 8.3
(7 questions)
You must be in the “Play mode” for the
slideshow for hyperlink to work.
Slide
of 25
© Copyright Pearson Prentice Hall
End Show
VIDEOS FOR ADDITIONAL INSTRUCTION
Section 8.3
None at this time.
Additional Videos for
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
SCI LINKS FOR CHAPTER
Additional Student SCI LINKS for CHAPTER 8
The NSTA-sponsored SciLinks Web site contains links to accurate and upto-date science
information on the Internet. Just click on the button below to go to the
SciLinks site at
www.scilinks.org and log in. Then, type in the SciLinks code for the topic
you want to
research. The following is a list of the SciLinks codes for this chapter.
Chapter 8: Chemical Equations and Reactions
Topic: Chemical Reactions
SciLinks code: HC60274
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
End of Chapter 8.3 Show
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.