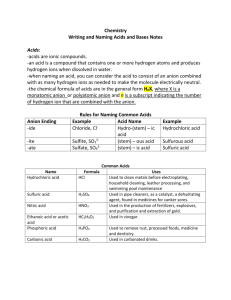
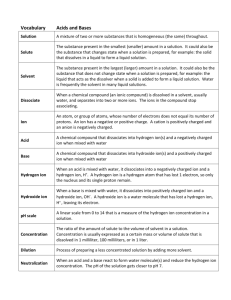
Acids and Bases
advertisement

Acids and Bases ICS Madrid 1 Revision When an acid reacts with a base, which compound(s) are formed? What is the name of H2SO4? What is the formula for phosphoric acid? Give four properties of acids. Give four properties of bases. 2 Acids and bases: revision What is the charge on the hydroxonium ion? If the hydrogen ion concentration is 10-10 M, is the solution acidic, alkaline, or neutral? If the [H+] in a solution is 1 x 10-1 mol/dm3, what is the [OH-]? 3 pH and concentration of strong acids and bases If the hydrogen ion concentration is 10-7 M, what is the pH of the solution? If the hydroxide ion concentration is 10-10 M, what is the pH of the solution? If the pH is 9, what is the concentration of the hydroxide ion? 4 If the pH is 6, what is the concentration of the hydrogen ion? How many free hydrogen ions are there in one litre of water? A litre of impure water has 10-4 mol of hydroxide ions. What is the concentration of hydrogen ions in this sample of water? 5 What is the concentration of hydrogen ions in a neutral solution? What is value of the ion-product constant for water? For a solution to be classified as acidic, the hydrogen-ion concentration must be _____ than the hydroxide-ion concentration. 6 questions A solution in which the hydroxide ion concentration is 1 x 10-4 M is ______. A solution in which the hydroxide ion concentration is 1 x 10-8 M is ______. The formula of the hydrogen ion is often written as ______. 7 The products of self-ionization of water are _____. is most basic: a) [OH1-] = 1 x 10-4 M, or b) [H1+] = 1 x 10-11 M? In a neutral solution, the [H1+] is ______. What is pH? Which 8 Which type of solution is one with a pH of 8? An indicator is what type of compound? In the reaction of aluminum bromide with ionised sodium bromide, which compound is the Lewis acid? 9 In the reaction of water with hydrogen ions, which compound is the Lewis base? What is an acid according to Arrhenius? What type of acid (mono-, di-, or triprotic) is sulphuric acid? Which hydrogens can ionise in a compound? 10 Which hydroxides can ionise in a compound? Which hydroxide compound yields the lowest concentration of hydroxide ions in aqueous solution: a) magnesium hydroxide, or b) potassium hydroxide? 11 Which acid is monoprotic: a) H2SO4, or b) CH3COOH? Which acid is dibasic a) H2SO4, or b) CH3COOH? 12 Which is an Arrhenius base: a) LiOH, or b) CH3COOH? What is an acid, according to Bronsted? Which of the following is a Bronsted-Lowry base, but not an Arrhenius base: a) sodium hydroxide, or b) ammonia? 13 Which compound can act as both a Bronsted-Lowry acid and a Bronsted-Lowry base: a) water, or b) sodium chloride? What type of acid or base is the ammonium ion? What is a conjugate acid made from? 14 What is transferred between a conjugate acid-base pair? What is the term used to describe a substance that can be both an acid and a base? the reaction: NH4+ + H2O NH3 + H3O+, water is acting as a(n) ______. In 15 the reaction: CO3- + H2O HCO3- + OH-, the carbonate ion is acting as a(n) _____. In What does amphoteric mean? Identify the Bronsted-Lowry bases in this reaction: H2S + H2O H3O+ + HS-. 16 Which of the following represents a Bronsted-Lowry conjugate acid-base pair: a) NH41+ and NH3 or b) SO32- and SO2? According to the Bronsted-Lowry theory, water acts as a base when it ______. 17 What are the acids in the following equilibrium reaction: CN- + H2O HCN + OHWhat is an “acid”, according to Lewis? Which acid is only a Lewis acid, and not any other kind: a) water, or b) boron trifluoride? 18 A hydrogen ion is what type of acid? A Lewis acid is a substance that can _____. What is the acid dissociation constant of a weak acid, if a concentration of 0.3 M gives a hydrogen ion concentration of 0.001 M? 19 What is the hydrogen ion concentration, if the acid dissociation constant is 1 x 10-6 M and the acid concentration is 0.01 M? What characterizes a “strong” acid or base? 20 The acid dissociation constant for an acid dissolved in water is equal to the ______. What is another name for the acid dissociation constant? What is the value of the acid dissociation constant for a weak acid? 21 How many ionization constants are associated with sulphuric acid? Which ionization constant is the largest for phosphoric acid? 22 Which of the following acids can be concentrated, but is always weak: a) ethanoic acid b) nitric acid? for HF = 6.7 x 10-4. If we have a 0.1 M solution, then [HF] is _______ than [H+] x [F-]. Ka 23 A 0.12 M solution of an acid that ionises only slightly in solution would be termed ___ and ___. Which of the following pairs consists of a weak acid and a strong base: a) sulphuric acid and sodium hydroxide, or b) acetic acid and sodium hydroxide? 24 What does “strong” mean when referring to acids and bases? Ethanoic acid ionizes in water as: 1% CH3COOH + H2O CH3COO- + H3O+ @ 99% The ethanoate ion (CH3COO-) is therefore a good _______ 25 A 12.0 M solution of an acid that ionises completely in solution would be termed ___ and ____. 26