Week 9 - SOW Year 10 Equilibria
advertisement

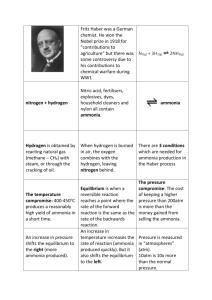
iGCSE Scheme of Work Year 10 Sept 2012 Equilibrium Lesson Lesson 1 Reversible reactions Learning Objectives understand that some reactions are reversible and are indicated by the symbol ⇌ in equations describe reversible reactions such as the dehydration of hydrated copper(II) sulfate and the effect of heat on ammonium chloride Activities Assessment Safety Starter-Write the one-way reaction and the reversible reaction equations on the board. Ask pupils to comment on differences between the two. MAIN Students practical on reversible reaction, see worksheet. Heat ammonium chloride and copper sulphate in separate test tubes. Show students the Animation C3 3.6 ‘Reversible energy’ from Additional Science CD ROM Pupils make notes from board including change in rates of forward and reverse reactions to equilibrium. Homework :Eye Ask students to find one further example of an equilibrium reaction and write a word and symbol equation to represent it. protection should be worn at all times. As acidic hydrogen chloride gas and alkaline ammonia gas are produced, the mineral plug must be used and the reaction should be carried out in a well ventilated room. Ammonium chloride is harmful – CLEAPSS Hazcard 9 Plenary True or false – Pupils use ‘show me boards to answer questions.’ All chemical reactions are reversible. [False] A closed system has no mass change. [True] Equilibrium can only happen in closed systems. [True] A double-headed arrow in an equation shows that it is a non-reversible reaction. [False] In a reversible reaction, reactants make products and products make reactants. [True] Show powerpoint. (5 minutes) Needs a more rigorous homework Other emphasis iGCSE Scheme of Work Year 10 Sept 2012 Equilibrium Lesson Lesson 2 Endothermic and exothermic Learning Objectives understand that chemical reactions in which heat energy is given out are described as exothermic and those in which heat energy is taken in are endothermic Activities Starter- Use a Bunsen burner to discuss the energy exchanges occurring between the reaction and the surroundings. Discuss this and the opposite possibility and the terms exothermic and endothermic reactions. Practical Pupils use coffee cup calorimeter as described on sheet. Do this for zinc/copper sulphate and endothermic expt. . Notes Pupils write notes on endo/exo. They need to understand the relationship with reversible reactions. Plenary- Use e-science ppt presentation Assessment Safety Students could list one exothermic reaction and one endothermic reaction that they could not live without and state why A more rigorous homework is needed here Other emphasis iGCSE Scheme of Work Year 10 Sept 2012 Equilibrium Lesson Learning Objectives Activities Assessment Safety STARTER Video – Show RSC video about the industrial production of ammonia. Use focus sheets. Homework – Fritz Haber worksheet Lesson 3 Making Ammonia understand that nitrogen from air, and hydrogen from natural gas or the cracking of hydrocarbons, are used in the manufacture of ammonia describe the manufacture of ammonia by the Haber process, including the essential conditions: i a temperature of about 450°C ii a pressure of about 200 atmospheres iii an iron catalyst understand how the cooling of the reaction mixture liquefies the ammonia produced and allows the unused hydrogen and nitrogen to be recirculated MAIN Introduce the production of ammonia and the benefits to society of ammonia and its compounds. Give pupils notes on the Haber process including equations and state conditions for all processes. Show students the Simulation C2 3.7 ‘Making Ammonia’ and allow them to explore the resource if possible. Pupils to use their book and the e-science video to complete the Haber process flow chart. PLENARY Questions and answers – Give each student a slip of paper (about A5 size). Ask them to write a question and its answer on the paper about the Haber process and reversible reactions, cut each answer free from the question and give them to you. Now give a question and answer to each student but they should not match. Read the first question out, the student with the correct answer should read it out, then read their question and so on. Other emphasis iGCSE Scheme of Work Year 10 Sept 2012 Equilibrium Lesson Learning Objectives Activities Assessment Safety Starter Needs exam question H/W Lesson 4 More about the Haber process, Conditions for equilibrium. understand the concept of dynamic equilibrium predict the effects of changing the pressure and temperature on the equilibrium position in reversible reactions. True or false questions about the Haber process could use show me boards. The t/f questions are on e-science ppt, C2 5.3 Main Give the students data for the yield of the Haber process at different temperatures and different pressures. Students could then plot two graphs to show the trend in yield as these variables change. Higher attaining students could have two x-axis scales describe the and plot onto one graph. They could then be manufacture of encouraged to use the graphs to make conclusions ammonia by the Haber process, about how temperature and pressure affects this including the reaction. Students could then be encouraged to essential conditions: explain these trends using information from the Student Book, related to their earlier work on energy i a temperature of changes in reactions. about This could be in groups, who then present their work 450°C or as individual Q + A. ii a pressure of about 200 atmospheres iii an iron catalyst describe the use of ammonia in the manufacture of nitric acid and fertilisers Discuss and makes notes on uses of ammonia Plenary C2 5.3 activity sheet: The yield in the Haber process. Other emphasis iGCSE Scheme of Work Year 10 Sept 2012 Equilibrium Lesson Lesson 5 Learning Objectives Activities Test Old AQA test is fine here Assessment Safety Other emphasis