Observations of Substances
advertisement

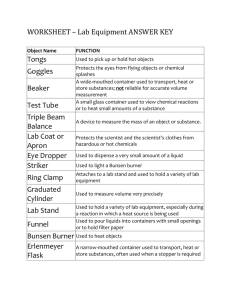
Intro to Chemistry Chem1020 Lab Observations of Substances Chemistry Department Minneapolis Community & Technical College 1 Overview • Part I Observing physical properties of elements and compounds • Part II Observing physical and chemical changes of various substances A. Changes caused by fire or heat B. Changes caused by mixing with other substances 2 Part I. Elements and Compounds Observing and recording physical properties (physical states, and colors) of elements and compounds Element: A substance that cannot be broken down into other substances by chemical means and contains only one element symbol in its formula. Compound: A substance that can be broken down into elements by chemical means and contains more than one element symbol in its formula. 3 Part I: Cautions! • Do not open any containers! Some substances are hazardous. • When describing a color, try your best. o If a gas or a liquid does not have any color, you should record colorless instead of clear or white. On the other hand, a solid can be recorded as having a white color. o Note the subtle difference between the colors of element iron and element carbon. 4 Part II. Physical vs. Chemical Changes Observing physical and chemical changes of various substances • Physical changes do not cause a change in the composition of a substance. Examples include melting, boiling (or evaporation), sublimation, etc. • Chemical changes do result in the formation of new substance(s) from the original substance(s). The observation of one or more of the following events may indicate the occurrence of a chemical change: o o o o Color change Formation of solid (precipitate) from liquid mixture Formation of gaseous product (bubbles, effervescence) Burning or combustion 5 Part II A Observing changes of substances caused by fire or indirect heat • Your instructor will first demonstrate with ammonium chloride (NH4Cl) (performed in fume hood) and a copper wire • Then you will study the following substances in the order: 1. 2. 3. Use the attached dropper to dispense a liquid sample Use a metal spatula to dispense a solid sample 6 Part II A 1. Add one drop of liquid (water or isopropyl alcohol), or a small amount of sugar (the relative amount shown on the right) onto the aluminum foil on the setup. 2. Approach (not touch) a lit stick to the surface of the substance. Does that substance catch on fire? If yes, is it a physical or chemical change? Record the observation. 7 Part II A 3. If there is no more fresh sample left from the previous procedure, add one drop of liquid or a small amount of solid onto the same aluminum foil. 4. Using a Bunsen burner, heat the sample on the aluminum foil from underneath. Wait until you observe some changes. Is it a physical of chemical change? A Bunsen Burner How to light it up? See the next slide. • After you are done with everything in this part, discard the used aluminum foil into a designated container. 8 How to Light up a Bunsen Burner? An Important Skill in a Chem Lab 1. Connect the burner and the gas valve with a rubber hose. 2. Gently close the gas valve of the burner. 5. Approach a lit stick or a striker to the top of the barrel. 4. Turn to open the gas valve. Meanwhile quickly turn on the gas valve of the burner. A striker 3. Gently close the air valve of the burner. Hottest spot 6. Turn on the air valve until you see two blue cones formed in the flame. 9 Part II B Observing changes of substances caused by mixing five pairs of chemicals. One of them, the fourth reaction, is shown below: Used and rinsed test tubes (pointing up) Waste container Find clean test tubes on your bench. Use tube #4 for this 4th reaction. A pair of chemicals 10 Part II B Mixing Chemicals 1. Choose the correct test tube. Add the required amount of each of the two chemicals in that reaction. 2. Make sure not to let the tip of the dropper touch the test tube (especially the inside) to avoid contamination. Put the dropper back to its original container. 3. Mix the chemicals by gently plucking the bottom of the test tube. Do you observe any signs of a chemical reaction? Record the observations. 11 Part II B. Waste Disposal • Don’t mix wastes, or dump them into the sink!!! • Empty the content from the test tube into the designated and properly labeled waste container which should be at the same station. • Rinse the test tube with water and dispose of the rinse into the same waste container. Waste container • Put the used test tubes in the test tube rack for further cleaning. Distilled water for rinsing used test tubes 12 • Wipe your bench with wet sponge. • Ask your instructor to sign you out….then you are ready to leave. • Finish the lab report (including both data sheet and postlab questions) after the lab. Feel free to ask instructors questions, but never ever copy other students’ answers. • Turn in the lab report the next time when you are expected to come to the lab. • Don’t forget to prepare for the next lab before you come next time. 13