AstronomicalSpectroscopy
advertisement

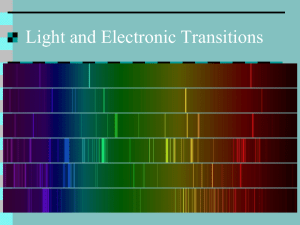
Spectroscopy The study of light emissions and absorptions The Visible Spectrum Newton’s Colour Wheel Spectral Colours Dispersion of White Light A Rainbow Creating a Spectrum Atomic Emission Lines • An emission line is formed when an electron makes a transition from a particular discrete energy level of an atom, to a lower energy state, emitting a photon of a particular energy and wavelength. • A spectrum of many such photons will show an emission spike at the wavelength associated with these photons. Atomic Emission Principles • When the electrons in the element are excited, they jump to higher energy levels. • As the electrons fall back down, and leave the excited state, energy is reemitted, the wavelength of which refers to the discrete lines of the emission spectrum. Electron Excitation The Hydrogen Atom Hydrogen Tube and Emission Bands Emissions from an elemental gas Emission Spectrum - H Emission Spectrum - He Emission Spectrum - Na Emission Spectrum - Fe Fireworks • Red - Strontium and Lithium salts • Orange - Calcium salts • Yellow - Sodium salts Fireworks • Green - Barium salts • Blue - Copper salts • Gold - incandescence of iron and charcoal • White - white hot metals Spectral Lamps • Sodium Lamp • Potassium Lamp • Cadmium Lamp Spectral Lamps • Helium Lamp • Thallium Lamp • Neon Lamp Qui c kTim e™ and a TIFF (Unc ompres s ed) dec ompres sor are needed to see thi s pi c ture. Spectral Lamps • Zinc Lamp • Rubidium Lamp • Mercury Sulfide Lamp Atomic emissions beyond the visible spectrum • Note that the emission extends over a range of frequencies. • The term often refers to the visible light emission spectrum, although it extends to the whole electromagnetic spectrum, from the low energy radio waves up to high energy gamma rays. Atomic Absorption Lines • An absorption line is formed when an electron makes a transition from a lower to a higher discrete energy state, with a photon being absorbed in the process. • These absorbed photons generally come from background continuum radiation and a spectrum will show a drop in the continuum radiation at the wavelength associated with the absorption. Comparing different spectra Astronomical Spectroscopy • Involves the scientific exploration and analysis of the properties of distant objects • Involves the observation of spectra at very high spectral resolutions Astronomical Spectroscopy • Chemical elements can be detected in astronomical objects by their emission lines and absorption lines • The shifting of spectral lines can be used to measure the redshift or blueshift of distant or fast-moving objects • Helium was first discovered by spectral analysis of light from the Sun Absorption Spectrum of the Sun Hydrogen Absorption Spectrum Origins of different Spectra The Sun - A Nuclear Reactor Formation of Deuterium as per The Big Bang Hydrogen Fusion in Stars Nuclear Fusion to form Helium - another possible mechanism Nucleosynthesis in a Massive Star Spectral Classes of Stars