File
advertisement

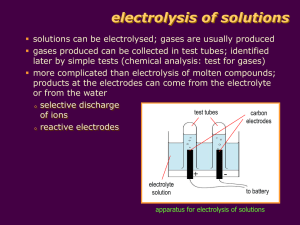
Academic class : Bachelor’s in Petroleum & Natural Gas Engineering Batch 12PG By Engr. Asadullah Memon B.E (Petroleum and Natural Gas) Electro Chemistry Electrolysis Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor (a metal or a semiconductor) and an ionic conductor (the electrolyte), and which involve electron transfer between the electrode and the electrolyte in solution. If a chemical reaction is driven by an external applied voltage, as in electrolysis, or if a voltage is created by a chemical reaction as in a battery, it is an electrochemical reaction. In contrast, chemical reactions where electrons are transferred between molecules are called oxidation/reduction (redox) reactions. Electrochemistry concerns the effects of electricity on chemical changes. Electro Chemistry Electrolysis Types of electro chemical cells There are two kinds electrochemical cells. 1. Electrochemical cells containing nonspontaneous chemical reactions are called electrolytic cells. 2. Electrochemical cells containing spontaneous chemical reactions are called voltaic or galvanic cells. Electro Chemistry Electrolysis Electrical Conduction Metals conduct electric currents well in a process called metallic conduction. In metallic conduction there is electron flow with no atomic motion. In ionic or electrolytic conduction ionic motion transports the electrons. Positively charged ions, cations, move toward the negative electrode, cathode. Negatively charged ions, anions, move toward the positive electrode, anode. Electro Chemistry Electrolysis Electrodes The following convention for electrodes is correct for either electrolytic or voltaic cells: The cathode is the electrode at which reduction occurs. The cathode is negative in electrolytic cells and positive in voltaic cells. The anode is the electrode at which oxidation occurs. The anode is positive in electrolytic cells and negative in voltaic cells. Electro Chemistry Electrolysis In general, electrochemistry deals with situations where oxidation and reduction reactions Oxidation (Loss of Electron). Reduction (Gain of Electron). Electrochemical reactions are oxidation-reduction reactions. The two parts of the reaction are physically separated. The oxidation reaction occurs in one cell. The reduction reaction occurs in the other cell. Electrolyte & Electrolysis Electrolytes are electrovalent substances that form ions (Cation & Anion) in solution which conduct an electric current The Phenomenon of decomposition of an electrolyte by passing electric current through its solution is known as Electrolysis. Examples: Electrolysis of Hydrochloric acid (HCL) Electrolysis of Sodium Chloride (Nacl) Electrolytic Cell Faraday’s Law of Electrolysis In 1834, Studied the quantitative aspect of Electrolysis. (Existence of Electrons) First law: The amount of a given product liberated at an electrode during electrolysis is directly proportional to the quantity of electricity which passes through the electrolyte solution. Second law: When the same quantity of electricity passes through solutions of different electrolytes, the amount of substances liberated at the electrodes are directly proportional to their chemical equivalents (Z) Importance of first law of electrolysis: Estimate, 1. The value of electrochemical equivalents of different substances. 2. The masses of different substances produced by passing a known quantity of electricity through their solution. Importance of Second law of electrolysis: Estimate, 1. Equivalent weight of metal. 2. Unit of electric current. 3. Avogadro’s number Electrolytic Conductance Conductance is the measure of the ease with which current can flow in the material (i.e. wire). Depends on the physical parameters of the material (length, area of cross section) as well as the conductivity. Conductivity, a measure of a material's ability to conduct an electric current (Electrical). Conductivity (electrolytic), a measurement of an electrolytic solution, such as water, HCL etc… The conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct electricity. It is the reciprocal of Resistivity. The SI unit of conductivity is siemens per meter (S/m). Conductivity Measurement The electrical conductivity of a solution of an electrolyte is measured by determining the resistance of the solution between two flat or cylindrical electrodes separated by a fixed distance. An alternating voltage is used in order to avoid electrolysis. The resistance is measured by a conductivity meter. Typical frequencies used are in the range 1-3 kHz. Uses of Conductivity Conductivity measurements are used extensively in many industries. For example, 1. To monitor quality in public water supplies, 2. In hospitals 3. In boiler water 4. To determine the amount of total dissolved solids (T.D.S.) 5. Petroleum 6. Iron and steel 7. Food Processing FACTORS AFFECTING ON ELECTROLYTIC CONDUCTANCE (1) Nature of electrolyte : The conductance of an electrolyte depends upon the number of ions present in the solution. Therefore, the greater the number of ions in the solution the greater is the conductance. The number of ions produced by an electrolyte depends upon its nature. The strong electrolytes dissociate almost completely into ions in solutions and, therefore, their solutions have high conductance. On the other hand, weak electrolytes, dissociate to only small extents and give lesser number of ions. Therefore, the solutions of weak electrolytes have low conductance. (2) Temperature : The conductivity of an electrolyte depends upon the temperature. With increase in temperature, the conductivity of an electrolyte increases. (3) Concentration of the solution Galvanic cell A Galvanic cell, or Voltaic cell, is an electrochemical cell that derives electrical energy from chemical reactions taking place within the cell. It generally consists of two different metals connected by a salt bridge, or individual half-cells separated by a porous membrane (As shown in Figure). Transport numbers The fraction of the total current carried by the cation or the anion is known as Transport number or Transference number or Hittorf’s number. It may be denoted by sets symbols like t+ and t– or tc and ta or nc and na” Where, ta = Current carried by an anion/Total current passed through the solution tc = Current carried by a cation/Total current passed through the solution evidently, ta + tc = 1 If v+ Represents the speed of migration of the cation and v_ that of the anion, the transport number of cation (t +) the transport number of anion (t_ ) Or The fraction of the total current carried by the ions are directly proportional to their velocities (HITTORF’S Rule). Hittorf’s Rule It state that “The loss in concentration around any electrode is proportional to the speed of the ion moving away from it Or Determination Methods of Transport Number The are two methods for determination of the transport number of an ion: 1. 2. HITTORF’S METHOD MOVING BOUNDARY METHOD 1. HITTORF’S METHOD Calcutions: Case # 01: When electrodes are unattackable (Pt Electrodes are used) Case# 02: When electrodes are attackable (Ag Electrodes are used) 2. MOVING BOUNDARY METHOD Suppose the boundary moves a distant x from AA’ to BB’ for the passage of Q coulombs. All the ions, H+, passed through the boundary AA’. The amount of substances transported is then Q/F, of which t+Q/F are carried by the positive ion. If the volume between the boundaries AA’ and BB’ is V, and the concentration of HCl is c, then tQ / F Vc FVc t Q Problems: Example no 1 (Page # 711), Example no 2 (Page # 712) and Example no 3 (Page # 714) ( Do your self) (Book referred Physical chemistry by B s Bahl) Surface and interfacial tension In dealing with multiphase systems, it is necessary to consider the effect of the force at the interface. When two immiscible fluid are in contact (Liquid and Gas), the term surface tension is used to describe the forces acting on the interface. When the interface is between liquid, the acting forces are called interfacial tension. Surface tension is caused by the net inward pull on the surface molecule. The inward forces on the surface molecules minimize the surface area and form a drop. Unit is dyne/cm (CGS) and N/M (SI) Factors Effecting on Surface Tension: 1. 2. 3. Temperature Pressure Density Calculation: Capillary Rise Method Surface tension = h.r.d.g / 2 Where, h = Height of liq: in cap:, cm r = Radius of cap, cm d = Density of liquid, g/cm g = Accerated due to gravity, cm/sec2