MS PowerPoint
advertisement
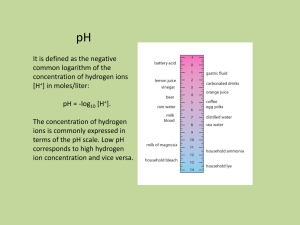
Faraday’s laws and applications of Kohlrausch’s laws Ramana Murthy. P Introduction Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor (a metal or a semiconductors) and an ionic conductor (the electolyte), and which involve electron transfer between the electrode and the electrolyte or species in solution. Alessandro Volta's discovery, in 1793, that electricity could be produced by placing two dissimilar metals on opposite sides of a moistened paper. In 1800, Nicholson and Carlisle, using Volta’s primitive battery as a source, showed that an electric current could decompose water into oxygen and hydrogen. By 1812, the Swedish chemist Berzelius could propose that all atoms are electrified, hydrogen and the metals being positive, the nonmetals negative. Humphry Davy prepared the first elemental sodium by electrolysis of a sodium hydroxide melt. Michael Faraday, to show that there is a direct relation between the amount of electric charge passed through the solution and the quantity of electrolysis products Chemical reactions where electrons are transferred between molecules are called oxidation/reduction (redox) reactions. In general, electrochemistry deals with situations where oxidation and reduction reactions are separated in space or time, connected by an external electric circuit to understand each process. Electron Transfer Reactions Electron transfer reactions are oxidation-reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by imposing an electric current. Therefore, this field of chemistry is often called ELECTROCHEMISTRY. Terminology for Redox Reactions OXIDATION :loss of electron(s) by a species; increase in oxidation number; increase in oxygen. REDUCTION: Gain of electron(s); decrease in oxidation number; decrease in oxygen; increase in hydrogen. OXIDIZING AGENT: Electron acceptor; species is reduced. REDUCING AGENT: Eelectron donor; species is oxidized. OXIDATION-REDUCTION REACTIONS Direct Redox Reaction Oxidizing and reducing agents in direct contact. Cu(s) + 2 Ag+(aq) ---> Cu2+(aq) + 2 Ag(s) Why Study Electrochemistry? • Batteries • Corrosion • Industrial production of chemicals such as Cl2, NaOH, F2 and Al • Biological redox reactions The heme group Classification of Conductors These may be divided into three main categories; they are: (I) gaseous (II) metallic or electronic (III) electrolytic. Gases conduct electricity with difficulty and only under the influence of high potentials or if exposed to the action of certain radiations. Metallic or electronic conductors : Conductors which transfer electric current by transfer of electrons, without transfer of any matter, are known as metallic or electronic conductors. Metals such as copper, silver, aluminum, etc., non-metals like carbon (graphite - an allotropic form of carbon) and various alloys belong to this class. Electrolytic conductors : (a) Conductors like aqueous solutions of acids, bases and salts in which the flow of electric current is accompanied by chemical decomposition are known as electrolytic conductors. b)The substances whose aqueous solutions do not conduct electric current are called nonelectrolytes. Solutions of cane sugar, glycerine, alcohol, etc., are examples of non-electrolytes. Fig. 1. Illustration of electrochemical terms Mechanisam of electrolytic conduction and electrolysis H+ + 2e2Cl- - 2e- 2NaCl(aq) + 2H2O(l) H2 (hydrogen gas at the (-)cathode). Cl2 (chlorine gas at the (+)anode). The overall reaction is 2Na+(aq) + 2OH-(aq) + Cl2(g)+ H2(g) Electrolysis of sodium chloride solution NaCl ↔ Na+ + ClH2O ↔ H+ + OHAt cathode H+ + e- → H 2H → H2 At Anode Cl- → Cl + e2Cl → Cl2 Electrolysis of copper sulphate solution using platinum electrodes CuSO4 ↔ Cu2+ + SO42H2O ↔ H+ + OHAt cathode At Anode Cu2+ + 2e- → Cu 2OH- → H2O + O + 2eO + O→O2 Some more examples of electrolysis Electrolyte Electrode Cathodic reaction Anodic reaction Aqueous acidified CuCl2 Pt Cu2+ + 2e-→ Cu 2Cl → Molten PbBr2 Pt Pb2+ + 2e- → Pb 2br → Br2 + 2e- Sodium chloride solution Hg 2Na+ + 2e-→2Na 2Cl- → Cl2 + Silver nitrate solution Pt Ag+ + e-→Ag 2OH- → 1/2 O2 + H2O + 2e- Sodium nitrate solution Pt 2H+ + 2e- → H2 2OH- → 1/2 O2 + H2O + 2e- Cl2 +2e- 2e- The decreasing order of discharge potential or the increasing order of deposition of some of the ions is given below: For cations: K+, Na+, Ca2+, Mg2+, Al3+, Zn2+, H+, Cu2+, Hg2+, Ag+ For anions: SO42-, NO3-, OH-, Cl-, Br-, I- TABLE OF STANDARD REDUCTION POTENTIALS oxidizing ability of ion Eo (V) Cu2+ + 2e- Cu +0.34 2 H+ + 2e- H2 0.00 Zn2+ + 2e- Zn -0.76 To determine an oxidation from a reduction table, just take the opposite sign of the reduction! reducing ability of element Faraday’s laws of electrolysis The laws, which govern the deposition of substances (In the form of ions) on electrodes during the process of electrolysis, is called Faraday's laws of electrolysis. These laws given by Michael Faraday in 1833. Faraday's first law: It states that, the mass of any substance deposited or liberated at any electrode is directly proportional to the quantity of electricity passed. WαQ W = Mass of ions liberated in gm, Q = Quantity of electricity passed in Coulombs = Current in Amperes ( i ) × Time in second (t) Wαit W=Zit Where, Z = constant, known as electrochemical equivalent (ECE) of the ion deposited Faraday's second law: It states that, when the same quantity of electricity is passed through different electrolytes, the masses of different ions liberated at the electrodes are directly proportional to their chemical equivalents (Equivalent weights). WαE W1/W2 = E1/E2 or Z1it / Z2it or Z1/Z2 = E1/E2 (W = Zit) E α Z or E = FZ or E = 96500 × Z Faraday's law for gaseous electrolytic product for the gases, we use V = It Ve/96500 Where, V = Volume of gas evolved at S.T.P. at an electrode Ve = Equivalent volume = Volume of gas evolved at an electrode at S.T.P. by 1 Faraday charge conductance and its measurement Ohm’s law Metallic as well as electrolytic conductors obey Ohm’s law which states the strength of current (I) flowing through a conductor is directly proportional difference (V) applied across the conductor and is inversely proportional to the resistance (R ) of the conductor I = V/R R - Resistance in V/A = Ω (Ohm) V - Voltage or potential difference in Volts, V I - Current in Amperes, A If a material has a resistance of 1 Ω, it means that when applying a potential difference of 1 V, the current in the material is 1 A. For metals: Ohm’s Law R = V/I R: resistance Dimension: Ohm, Conductance is the ability of a material to pass electrons C=1/R Specific conductance or conductivity The resistance of any conductor varies directly as its length (l) and inversely as its cross sectional area (a), i.e., R α 1/a or R = ρ 1/a , Here ρ = specific resistance If l = 1 cm and a = 1 cm2, then R=ρ Κ= 1/ρ, Κ = kappa - the specific conductance ρ = a/l. R or 1/ρ = 1/a.1/R K = 1/a×C (1/z = cell constant) Specific conductance = cell constant x Conductance The unit of specific conductance is ohm-1 cm-1. Specific conductance or conductivity - + - anode Solution Cathode + 1c m 1c m Representation of specific conductance Specific conductance depend on the nuber of ions present in unit volume (1 ml ) solution Equivalent conductance (/\) To understand the manning of equivalent conductance, imagine a rectangular trough with two opposite sides made of metallic conductor (acting as electrodes) exactly 1 cm apart, If 1 cm3 (1 mL) solution containing 1 gram equivalent of an electrolyte is places in this container is measured. /\ = KV In case, if the concentration of the solution is c g equivalent per litre, then the volume containing 1 g equivalent of the electrolyte will be 1000/C. So equivalent conductance /\ k 1000/c /\ = k × 1000/N Where N = normality The unit of equivalent conductance is ohm-1 cm-2 equi-1. One of the factors on which the conductance of an electrolytic solution depends is the concentration of the solution. In order to obtain comparable results for different electrolytes, it is necessary to take equivalent conductances. Equivalent conductance is defined as the conductance of all the ions produced by one gram equivalent of an electrolyte in a given solution. 1 cc m 1c 1 cm Representation of Equivalent conductance Molar conductance The molar conductance is defined as the conductance of all the ions produced by ionization of 1 g mole of an electrolyte when present in V mL of solution. It is denoted by. Molar conductance Λ m = k ×V Where V is the volume in mL containing 1 g mole of the electrolyte. If c is the concentration of the solution in g mole per litre, then Λ m = k × 1000/c It units are ohm-1 cm2 mol-1. Equivalent conductance = (Molar conductance)/n Where n = (Molecular mass) / (Equivalent mass) Effect of dilution on equivalent conductance Conductance’s of electrolytes of different type Kohlrausch’s law of independent ionic mobilities At time infinite dilution (m) , the molar conductivity of an electrolyte can be expressed as the sum of the contributions from its individual ions Λ∞m = v+ λ∞ + + v- λ∞v+ and v- are the number of cations and anions per formula unit of electrolyte respectively and, λ∞+ and λ∞- are the molar conductivities of the cation and anion at infinite dilution respectively Applications of Kohlrausch's law Determination of Λ∞m for weak electrolytes Determination of the degree of ionization of a weak electrolyte Determination of the ionization constant of a weak electrolyte Determination of the solubility of a sparingly soluble salt Determination of Λ∞m for weak electrolytes The molar conductivity of a weak electrolyte at infinite dilution (Λ∞m) cannot be determined by extrapolation method. However, Λ∞m values for weak electrolytes can be determined by using the Kohlrausch's equation. Λ∞CH3 COOH = Λ∞CH3COONa + Λ∞HCI - Λ∞NaCI Determination of the degree of ionization of a weak electrolyte The degree of ionization is given by ac = Λcm /Λ∞m = Λcm / ( v+ λ∞+ + v- λ∞- ) Thus, knowing the value of Λcm, and Λ∞m (From the Kohlrausch's equation), the degree of ionization at any concentration (ac) can be determined. Determination of the ionization constant of a weak electrolyte ( K ) = C(Λcm / Λ∞m )2 / [ 1 - ( Λcm / Λ∞m )] = C(Λcm)2 / Λ∞m - Λcm ) We know Λ∞m and Λcm at any concentration, the ionisation constant (K) of the electrolyte can be determined. Determination of the solubility of a sparingly soluble salt the molar conductivity of a sparingly soluble salt at infinite dilution Λ∞m = V+λ∞+ + V-λ∞Λ∞salt = 1000 ksalt / Cm Cm = 1000 ksalt / ( V+λ∞+ + V-λ∞- ), Cm is the molar concentration of the sparingly soluble salt in its saturated solution. Thus,Cm is equal to the solubility of the sparingly soluble salt in the mole per litre units. The solubility of the salt in gram per litre units can be obtained by multiplying Cm with the molar mass of the salt. Zn --> Zn2+ + 2e- Cu2+ + 2e- --> Cu Oxidation Anode Negative Reduction Cathode Positive <--Anions Cations--> •Electrons travel thru external wire. •Salt bridge allows anions and cations to move between electrode compartments. RED CAT Charging a Battery When you charge a battery, you are forcing the electrons backwards (from the + to the -). To do this, you will need a higher voltage backwards than forwards. This is why the ammeter in your car often goes slightly higher while your battery is charging, and then returns to normal. In your car, the battery charger is called an alternator. If you have a dead battery, it could be the battery needs to be replaced OR the alternator is not charging the battery properly. H2 as a Fuel Cars can use electricity generated by H2/O2 fuel cells. H2 carried in tanks or generated from hydrocarbons