Molecular Motion in Liquid
advertisement

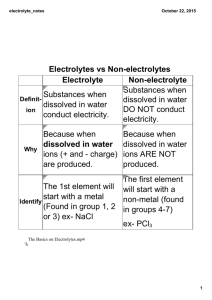
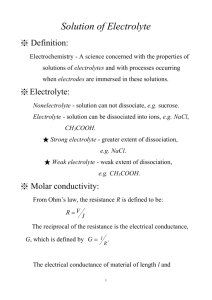
Chapter 21 Molecular motion in liquids Summary of Midterm I • • • • • • • • • Class average 78%; Lowest 25%; highest 96% A+ 12 students A 17 students A15 students B+ 6 students B 4 students B6 students F 3 students 21.5 Experimental results • Measuring techniques: NMR, ESR, inelastic neutron scattering, etc. • Big molecules in viscous fluids typically rotate in a series of small (5o) steps. • Small molecules in nonviscous fluid typically jump through about 1 radian (57o). • For a molecule to move in liquid, it must acquire at least a minimum energy to escape from its neighbors. • The change in density has pronounced influence on the viscosity. • The probability that a molecule has at least an energy Ea is proportional to e-Ea/RT. • Viscosity, η, is inversely proportional to the mobility of the particles, η ∞ eEa/RT Temperature dependence of the viscosity of water This is opposite to the gases, where the viscosity increases with temperature 24.6 The conductivities of electrolyte solutions • Conductance (G, siemens) of a solution sample decreases with its length l and increases with its cross-sectional area A: G • kA l k is the conductivity (Sm-1). Molar conductivity, Λm, is defined as: m k c c is the molar concentration • Λm varies with the concentration due to two reasons: • Based on the concentration dependence of molar conductivities, electrolytes can be classified into two categories: 1. Strong electrolyte: its molar conductivity depends only slightly on the molar concentration. 2. Weak electrolyte: its molar conductivity is normal at diluted environment, but falls sharply as the concentration increases. Strong electrolyte • Strong electrolyte is virtually fully ionized in solution, such as ionic solid, strong acids and bases. • According to Kohlrausch’s law, the molar conductivity of strong electrolyte varies linearly with the square root of the concentration: m 0m • c1 / 2 Λ0m , the limiting molar conductivity, can be expressed as the sum of contributions from its individual ions: 0m v v where v+ and v- are the numbers of cations and anions per formula unit. (For example: HCl: v+ = 1 and v- = 1; MgCl2, v+ = 1 and v- = 2) Weak electrolyte • Weak electrolytes are not fully ionized in solution, such as weak acids and bases. • Degree of ionization (α): defined as the ratio of the amount of ions being formed in the solution and the amount of electrolyte added to the solution. • For the acid HA at a molar concentration c, [H3O+] = αc, [A-] = αc , [HA] = c –αc K a a 2c 1/ 2 4c 1 1 Ka • Since only fraction, α, of electrolyte is actually presents as ions, the measured conductivity Λm, is given by: Λm = αΛ0m Ostwald’s dilution law 1 1 0 m m mc K a 0m 2 24.7 The mobility of ions • Drift speed (s): the terminal speed reached when the accelerating force is balanced by the viscous drag. • Accelerating force induced by a uniform electric field (E = Δø/l): F = z e E = z e Δø/l • Friction force (Stokes formula) Ffric = (6πηa)s, hydrodynamic radius a is the • Introducing a new quantity, the mobility of an ion: u • Then ze 6 a Mobility and conductivity • λ = z u F ( λ is an ion’s molar conductivity) • For the solution: Λ0m = (z+u+v+ + z-u-v-) F Transport numbers • Is defined as the fraction of total current carried by the ions of a specified type. I t I • t I I The limiting transport number, t0±, is defined for the limit of zero concentration of the electrolyte solution. t 0 z v u z v u zv u v t v v 0 The measurement of transport numbers • Moving boundary method • Indicator solution • Leading solution z clAF t It Conductivities and ion-ion interactions • To explain the c1/2 dependence in the Kohlrausch law.