Answers - Chemistry Courses: About
advertisement
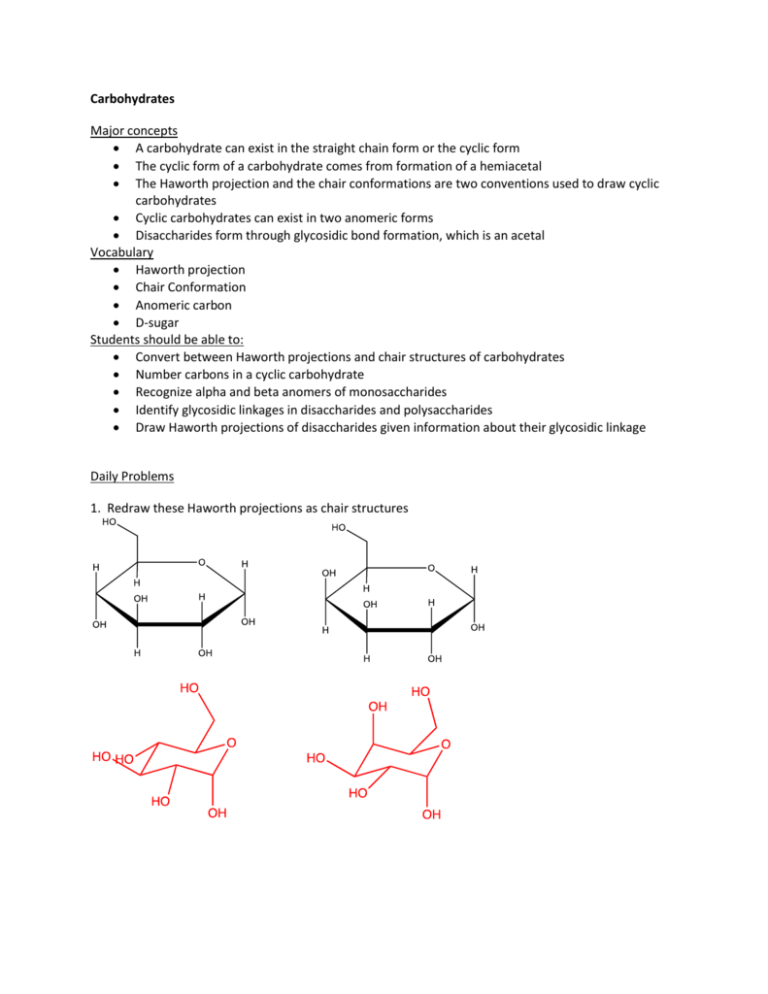
Carbohydrates Major concepts A carbohydrate can exist in the straight chain form or the cyclic form The cyclic form of a carbohydrate comes from formation of a hemiacetal The Haworth projection and the chair conformations are two conventions used to draw cyclic carbohydrates Cyclic carbohydrates can exist in two anomeric forms Disaccharides form through glycosidic bond formation, which is an acetal Vocabulary Haworth projection Chair Conformation Anomeric carbon D-sugar Students should be able to: Convert between Haworth projections and chair structures of carbohydrates Number carbons in a cyclic carbohydrate Recognize alpha and beta anomers of monosaccharides Identify glycosidic linkages in disaccharides and polysaccharides Draw Haworth projections of disaccharides given information about their glycosidic linkage Daily Problems 1. Redraw these Haworth projections as chair structures 2. Redraw these chair structures as Haworth Projections. 3. Which of these compounds is -Dgalactose? How do you know? The hydroxyl group that is part of the acetal is down, making this Dgalactose. 4. Two glucose molecules can be linked together through a glycosidic bond to make a disaccharide. An organic chemist recognizes that a glycosidic bond is just another name for an acetal. The glycosidic bond can be classified by the direction of the bond made—if the bond is “down,” it is labeled an glycosidic bond; if it is “up,” it is labeled a -glycosidic bond. Most disaccharides also contain a hemiacetal, and the direction of the hemiacetal OH group determines which “anomer” the disaccharide is: the anomer has its hemiacetal hydroxyl group “down” and the anomer has its hemiacetal hydroxyl group “up.” H OH 4 H HO HO O 2 3 H H 1 OH H H OH 4 O HO H 2 3 H H O 1 OH OH H A. When two glucose molecules are joined with a glycosidic bond like the one shown above, the disaccharide is called maltose. What type of glycosidic bond is found in maltose? α-glycosidic bond B. Which anomer of maltose is shown above? α (14) linkage 5. Cellobiose is a disaccharide very much like maltose, except it has a (14) glycosidic linkage. Draw the structure of the alpha anomer of cellobiose, using maltose from problem 4 as a guide. Then draw the beta anomer of cellobiose. α (14) glycosidic linkage (14) glycosidic linkage 6. Polysaccharides are similar to disaccharides, but many carbohydrate units are connected. Examples of polysaccharides made up entirely of glucose include starch, glycogen, and cellulose. Maltose, starch, and glycogen differ from cellulose in that humans can digest the first three, but not cellulose, because we don’t have enzymes that recognize a feature of cellulose. What structural feature do you think is responsible for enzyme discrimination between starch and cellulose? H OH H O O H H OH starch HO H OH H H O O H H HO H OH OH H H O O O HO H cellulose H OH H H OH H H OH H O H O O H O O HO H H O HO OH OH H H H O HO OH H OH H H H Starch and other polysaccharides that we can digest have α glycosidic linkage while cellulose has glycosidic linkage. 7. Glucose can exist in the straight chain form, but because it contains both hydroxyl groups and an aldehyde, it often cyclizes on itself to form a hemiacetal. On the ring structures, add numbers to the carbons that match the numbers on the straight chain compound. 6 5 4 6 1 4 3 2 5 1 3 2 8. Draw a mechanism for formation of the six-member cyclic glucose under acid catalysis. (Hint: what functional group is formed?) hemiacetal