spectroscopy-flame tests
advertisement

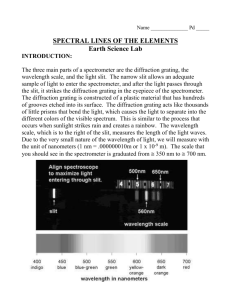
Spectroscopy - Flame Tests Objectives: • To explain the origins of a line (emission and absorption) spectrum • To observe the flame emission colors from excited metal ions • To identify an element from its emission spectrum Suggested Grade Level(s): Ninth through twelfth grade Subject areas: Chemistry Astronomy Earth Science Timeline: One class period (1 hour) National Education Standards: 6.2. Physical Science (grades 9-12) Structure of atoms 6.5. Science and Technology (grades 9-12) Understanding about science and technology Background: This can also be used as an introduction studying the composition of planets and stars as well as studying the physics of color and light. If used as an introduction to composition of planets and stars student should be familiar with using a spectroscope and understand what information can be obtained from the spectroscope. This activity can also be used as a cumulating lesson after students have learned about the structure of the atom, electrons and energy levels as well as a basic understanding of the electromagnetic spectrum. Materials: Report Sheet Platinum or Nichrome wire Concentrated HCl Flame source Watchglass Crystals of calcium chloride CuCl2, NaCl, BaCl2, LiCl, SrCl2 and KCl salts Cobalt glass plate Lesson: INTRODUCTION The current model of the atom includes a nucleus, comprised of protons and neutrons, surrounded by electrons. From interpretations of the atomic absorption and emission spectra of elements, and from a detailed quantum study of electron behavior, it is generally agreed that the energy of each electron in an atom is quantized. This means that an electron in an atom may have only certain discrete energies. When an electron absorbs or emits energy, it can only absorb or emit a “quantum” of energy. When an atom absorbs energy from a flame or an electric discharge, it absorbs the energy necessary to excite one of its electrons to a higher energy state. We say the atom is then in an excited state, Eλ. When the electron returns to a lowest energy state (ground state), it emits the same amount of energy previously absorbed, but in the form of one or more photons E (excited state) ________________ _______e-___________ E photon ________ E photon E (grounded state) _____e-___________ __________________ The energy of the photon equals the difference between the two energy states for the electron and is inversely proportional to its wavelength. Since many energy states exist for electrons in a given atom, a large number of excited states are also possible. Therefore, when light is emitted from a large collection of excited atoms, a variety of electrons return to their ground state. When these emitted photons pass thorough a prism, a line spectrum is produced, where each lime on the spectrum corresponds to photons of fixed energy and wavelength. Each element exhibits its own characteristic lime spectrum because the electron energy states in its atoms are different from those in atoms of other elements. For example, the 11 electrons on sodium have a different set of electron energy states than do the 80 electrons in mercury or the 56 electrons in barium. Therefore when an electron in an excited sodium atom moves to a lower energy state, a photon is emitted. This photon has a different energy and wavelength from one that is emitted when an electron de-excites in mercury or a barium atom. The different wavelengths of the emitted photons produce different, but characteristic, colors of light. Light emitted from an excited sodium atom is characteristically yellow-orange, mercury emits a blue light, and barium emits a green light. In part A of this experiment, we will observe the characteristic colors emitted from several “excited” elements. Flame tests are used for identification. The probability of a given electron de-excitation process in an atom reflects the intensity of that line of given energy in the line spectrum- the more probable the de-excitation, the more intense the line. An analytical study of line positions and intensities in an emission spectrum will be made in parts B and C. 1. Part A: Flame test of various elements a. Clean the Test Loop. Dip the platinum or nichrome wire end of the test loop into concentrated HCl (caution: avoid contact with skin.) Heat the wire in the hottest region of the flame until there is no visible color. Repeat this cleaning procedure as necessary. b. Flame test for calcium in CaCl2. In a watchglass add a few crystals of calcium chloride, to 2 or 3 drops of concentrated HCl and stir. Dip the clean wire into the mixture and return it to the flame (view it through a spectroscope). Correlate the color of the flame with the wavelength of the emission using the chart on the Report Sheet. Check the appropriate section. c. Flame tests for Other Cations. Repeat parts 1 and 2 substituting CuCl2, NaCl, BaCl2, LiCl, SrCl2 and KCl salts. For KCl view the flame through a cobalt glass plate- it filters out wavelengths that interfere with those of the excited state 2. Part B: The Spectrum of an unknown element a. Determine the wavelengths in the Emission Spectrum of the Unknown. You will be given an unknown sample from one that was done in Part A. Identify the compound by comparing it to the results of Part A 3. Post Lab Questions a. Explain why the color of the ion in the flame tests differs? b. Over what (approximate) wavelength range is the emission of light from excited sodium atoms most sensitive to the eye? Hint: sodium vapor lamps are used for public lighting of streets, parking areas, etc c. Over what (approximate) wavelength range is the emission of light from excited mercury atoms most sensitive to the eye? Hint: mercury vapor lamps are used for public lighting of streets, parking areas, etc d. Aerial displays of fireworks are accompanied by many colors in the starbursts. • What is the source of the orange-yellow color? • What ion is responsible for the red color? Extensions: As an extension students can research how other sciences use this technology, like forensics, geology and\or biology. Evaluation: Students were evaluated based on the post lab questions as well as the results from part B of the lab. Resources: Space Foundation ‘Astronomy Principles for the Classroom’ June 25-29, 2007 Addendums: Addendums PRELABORATORY ASSIGNMENT SPECTROSCOPY NAME______________________ Date________________________ 1. Distinguish between an absorption spectrum and an emission spectrum. 2. The strong line in the calcium emission line spectrum occurs at 657.3 nm, to what color does this correspond? 3. What does it mean when we say that an atom is in an “excited state”? Data table: For each element, record the approximate wavelengths emitted by each ion. Wavelength in nanometers 740 Red Orange Yellow Green 520 565 590 625 Cyan 500 Blue 435 Violet Element 380