Outline Curriculum (5 lectures) Each lecture 45
advertisement
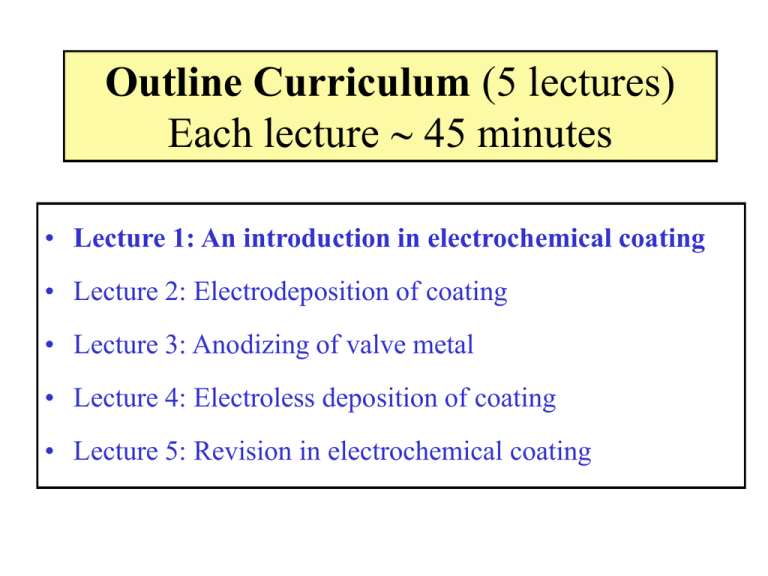
Outline Curriculum (5 lectures) Each lecture 45 minutes • Lecture 1: An introduction in electrochemical coating • Lecture 2: Electrodeposition of coating • Lecture 3: Anodizing of valve metal • Lecture 4: Electroless deposition of coating • Lecture 5: Revision in electrochemical coating Lecture 1 of 5 An Introduction In Electrochemical Coating Electrochemical Surface Engineering (Electrochemical Coating) • Is it about the deposition a coating onto surface, via electrochemical reactions. • The coating can be (a) metallic, (b) metal oxide or (c) conductive polymer. • Metallic coating: Electroplating • Metal oxide, conductive polymer: Anodizing • Electroless deposition Electrochemical Surface Engineering • • • • • An electro-chemical reaction Cathode: Metals/alloys coating Anode: Metal oxides Conductive solution: ionic species Transfer of electrons Electroplating of copper Anodizing • An electrolytic passivation process. • To form a thick oxide layer on a metal. • Metal oxide forms on the anode. Electroless deposition • Electroplating: consisting of two electrodes, electrolyte, and external source of current. • Electroless deposition: this process uses only one electrode and no external source of electric current. • Electroless deposition: the solution needs to contain a reducing agent so that the reaction can proceed: • Metal ion + Reduction solution Metal solid + oxidation solution Catalytic surface Definition: Electron transfer reactions • Oxidizing agent + n e- = Reducing agent • Oxidizing agents get reduced • Reducing agents get oxidized • Oxidation is a loss of electrons (OIL) OILRIG • Reduction is a gain of electrons (RIG) Industrial scale anodizing of Aluminium Example of anodizing Brush electroplating of gold onto stainless steel substrate Tin-Zinc coating onto steel substrate Benefits of electroplated metallic surfaces: 1. 2. 3. 4. Improved corrosion resistance. Improved wear resistance. Longer lifetime. Aesthetic surface finish. Optical micrograph of 21 mm PEO coating on Mg alloy: Optical micrograph of 12 mm PEO coating on Mg alloy: Porosity in electroless Ni-P deposits (<5 mm) on mild steel Log-log Porosity vs. thickness for electroless Ni-P deposits on steel % Porosity 100 10 1 1 Deposit thickness/mm 10 Electrochemical anodizing Transformation of Ti foil to TiO2 nanotubes Anodizing e.g. 10-100 V Competing reactions for the formation of TiO2 nanotubes Electrochemical formation of oxide Ti + 2H2O → TiO2 + 4H+ + 4eChemical dissolution of oxide TiO2 + 6F- + 4H+ → TiF62- + 2H2O Green electrolyte, CH3SO3H Anodizing of TiO2 nanotubes from Ti foil 100 nm 200 nm 100 nm 200 nm Surface microstructure Nanotubes Au-TiO2 vertically aligned array 1 mm 100 nm 100 nm Reflective nanocrystalline PbO2 Application: Solar heat absorber 20 Rotating Cylinder Reactor High throughput electrodeposition Cu-Sn alloys Rotating Cylinder Reactor High throughput electrodeposition Cu-Sn alloys Nanoparticles SiC in a nickel matrix Wear resistance coating Darker contrast: nanoparticle SiC 100 nm Ni-SiC coating Copper substrate 200 mm TEM image Nanotubes TiO2 in a nickel matrix Nanotubes TiO2 20 nm Nickel matrix 100 nm Electrodeposition of polypyrrole Stainless steel substrate Polypyrrole 1.0 cm 1.0 cm 25 Electrocatalysts for H2O electrolysis Nanocrystalline and amorphous Ni-Co alloys 0g Co 2 g 10 g 20 g 40 g 60 g 80 g 100 g 150 g 200 g 100g Ni 1.0 cm Co content in alloyed electrocatalyst increases More effective electrocatalyst to evolution oxygen 26 Large scale electrodeposition Thick film, multilayered Ni-Co on Fe substrate 200 μm Ni Ni Fe 20 cm Each tank = 5 Litres Co Multilayered - and -PbO2 α- and β-PbO2 β-PbO2 28 Thin film lead-acid battery Nanosized materials Nanosized material PbO2 + PbSO4 100 nm 29 Summary • Electrochemical coatings range from nanoparticles of metal on nanostructured, inorganic supports through to hard <100 mm Cr coatings on steel. • Applications include catalysts, fuel cell-, solar cell- and battery electrodes together with tribological/corrosion resistant coatings for electronic materials, transport and heavy engineering. • Plasma electrolytic oxidation uses the application of a high a.c. voltage to produce a hard, wear resistant oxide coating on light metals (such as Mg alloys) for automotive, aerospace and leisure. • Electroless Ni deposits (typically <20 mm in thickness) on steel or Al alloys are widely used in engineering applications for their corrosion and wear resistance. Thin coatings tend to have high porosity.