Polymer Synthesis CHEM 421 Living Polymerization
advertisement

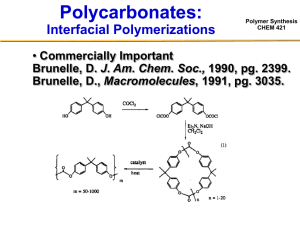
Polymer Synthesis CHEM 421 • Odian Book Chapter 3-15, 5-3 Polymer Synthesis CHEM 421 Living Polymerization Living Polymerization Neutral and Highly Reactive or Polymer Synthesis CHEM 421 Living Polymerizations Polymer Synthesis CHEM 421 • Living polymerizations are chain growth polymerizations which proceed in the absence of irreversible chain transfer and termination steps.* • Diagnostic Characteristic of Living Polymerizations – The reaction proceeds until all monomer is consumed. If more monomer is introduced then the polymerization will continue – The number average molecular weight, Mn, is a linear function of conversion. – The number of propagating chains (active centers) is constant and independent of conversion. – Mn can be controlled by the reaction stoichiometry. – Sequential monomer addition results in the preparation of block copolymers. – Resulting polymers will exhibit a narrow molecular weight distribution and the polymer must exhibit a Poisson distribution in molecular weight.** * Szwarc, M., Nature, 1956, 178, 1168. ** Flory, P. J., J. Am. Chem. Soc., 1940, 62, 1561. Living Polymerization Polymer Synthesis CHEM 421 • No termination or chain-transfer side reaction during polymerization –Control of molecular weight grams of monomer » M n= Moles of initiator –Narrow molecular weight distribution –Synthesis of block copolymers by sequential monomer addition –Control of polymer chain microstructure –End-group functionalization Initiation Polymer Synthesis CHEM 421 • Nucleophilic Initiation of Vinyl Monomers CH3 CH3CH2CH Li H2C CH CH Li Bu CH2 CH CH Li Li CH Li Anionic Polymerization of Styrene Polymer Synthesis CHEM 421 Anionic Polymerization of Styrene Polymer Synthesis CHEM 421 Anionic Polymerization of Styrene Polymer Synthesis CHEM 421 Anionic Polymerization of Styrene Polymer Synthesis CHEM 421 Initiators: Organolithiums • Soluble in hydrocarbons • Direct nucleophilic attack • No electron transfer (RLi)N N= 6,4,2 MW= g of monomer moles of initiator Polymer Synthesis CHEM 421 The Poisson Distribution in Molecular Weight Polymer Synthesis CHEM 421 • The constraints imposed on a living polymer require that the polymer exhibit a Poisson distribution in molecular weight.* Conversion PDI Time Mw/Mn DPw 1 1 DPn DPn Conversion Conversion & Mw/Mn as a Function of Time Scale of “Livingness” Polymer Synthesis CHEM 421 • Conversion should be linear with time in semi-logrithmic coordinates • Deceleration indicates termination or deactivation of catalyst. ln[M]o/[M]t Ln[M]o/[M] vs. Time kp/kt = 1.05 kp/kt = 10 kp/kt = 100 Living • When chain termination cannot be completely suppressed (kt > 0): - deviation from living behavior becomes more pronounced with time Time - The degree to which a reaction deviates at time, t, is proportional to the ratio of the rate of propagation to termination. End Group Functionalization Carboxylic acid terminated: Primary alcohol terminated: Polymer Synthesis CHEM 421 End Group Functionalization Polymer Synthesis CHEM 421 Monomers Polymer Synthesis CHEM 421 • Generally olefin w/ EWG or delocalizing groups Methyl acrylate (MA) Methyl methacrylate (MMA) t-Butyl methacrylate (tBuMA) Ion Pair Aggregation • Need to stabilize propagating anions Polymer Synthesis CHEM 421 Ion Pair Aggregation • Need to stabilize propagating anions Polymer Synthesis CHEM 421