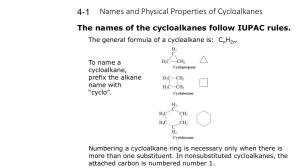
cycloalkanes
advertisement
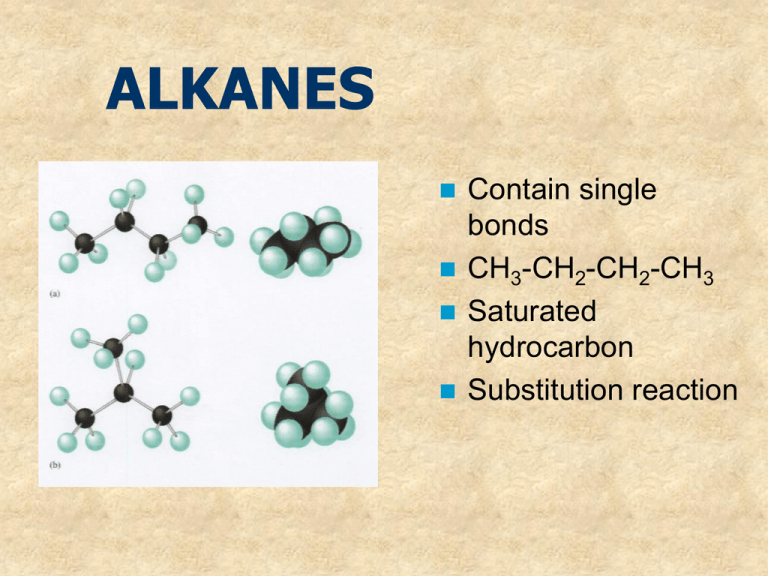
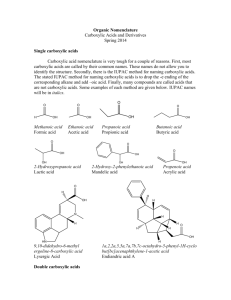
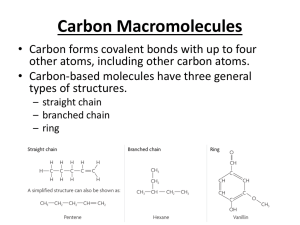
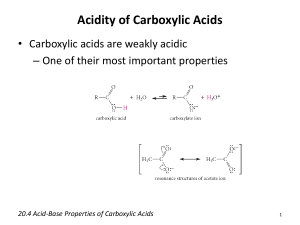
ALKANES Contain single bonds CH3-CH2-CH2-CH3 Saturated hydrocarbon Substitution reaction Cycloalkanes If the carbon chain that forms the straightchain hydrocarbon is long enough, the two ends coming together to form a cycloalkane. One hydrogen atom has to be removed from each end of the hydrocarbon chain to form the CC bond that closes the ring. Cycloalkanes therefore have two less hydrogen atoms than the parent alkane and a generic formula of CnH2n. Examples of cycloalkanes The smallest alkane that can form a ring is cyclopropane, C3H6, in which the three carbon atoms lie in the same plane. The angle between adjacent CC bonds is only 60° as seen in the model below: Molecular models of cycloalkanes: cyclohexane cyclobutane cyclopropane cyclopentane Name the following: The following compound, for example, is a derivative of pentane because the longest chain contains five carbon atoms: Thus, its name is 2-methylpentane Naming the cycloalkanes: Find the longest continuous loop of carbon atoms in the skeleton structure. Name the compound as a derivative of the cycloalkane with this number of carbon atoms: 3-C = cyclopropane 4-C = cyclobutane 5-C = cyclopentane Name the following cycloalkanes: Rings (or cyclic compounds) are composed of rings of carbon and sometimes oxygen or nitrogen. For example, cyclohexane has a ring of six carbon atoms. cyclopropane cyclobutane cyclopentane cyclohexanebromocyclohexane cyclohexene cyclohexane Alkenes Contain a double bond Unsaturated Addition reactions CH2=CH-CH2-CH3 1-butene Polymerize Alkynes WHAT IS AN ALKYNE ???? WHAT DOES IT LOOK LIKE ? WHAT IS IT’S STRUCTURE ? QUIZ A hydrocarbon with triple bonds. These make up the alkyne family Ethyne C2H2 H- C C - H Ethyne C2H2 WHICH ONE IS AN ALKYNE? It’s not this one. Try again You got it !!!!! HOW DO WE NAME IT ? Name the Alkane compound with an -yne ending for a triple bond. The alkane methane as an alkyne…. What is it’s name ? methyne The aromatic hydrocarbons: Benezene is good example: C6H6 Interior ring formed by three double C-C bonds Also called a benzene ring The carboxylic acids: Carboxylic acids contain the carboxyl group, which is a contraction of the carbonyl and hydroxyl names since carboxylic acids contain both groups attached to one carbon. Often written as: RCOOH or RCO2H IUPAC Nomenclature Carboxylic acids are named by replacing the -e of the alkane root name with -oic and adding the word acid. Substituents on the chain are named as usual. Some common acids: methanoic acid ethanoic acid propanoic acid 2-methylpropanoic acid IUPAC naming of acids: The table below gives common and IUPAC names for some of the simple carboxylic acids. IUPAC Name Common Name Formula methanoic acid formic acid HCOOH ethanoic acid acetic acid CH3COOH propanoic acid propionic acid CH3CH2COOH butanoic acid butyric CH3(CH2)2COOH pentanoic acid valeric CH3(CH2)3COOH Alcohols R–OH R= alkyl group Poisonous Good solvents CH3CH2CH2OH 1-propanol Ethers R-O-R/ If R=R/ symmetrical CH3-O-CH3 Dimethyl ether CH3-O-CH2CH3 Ethylmethyl ether Non-polar solvents ALDEHYDES O Carbonyl group –C— O H - C -H Methanal http://www.chem.uni.edu/~macmil la/mcmurry/mcmurry_chapter_19a /sld001.htm Esters Formed by a reaction of an acid and an alcohol. Conversion of acids into esters is called esterification. Ester structure Ketones General structure R—CO—R' Organic compounds, usually react AMINES Contain nitrogen R-NH2 CH3-NH2 Methylamine Polar substance