THE Macromolecules PowerPoint - Panhandle Area Educational
advertisement

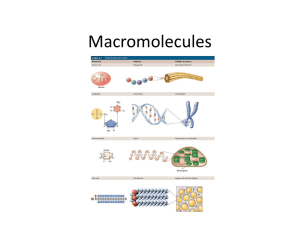
Biology Partnership (A Teacher Quality Grant) Macromolecules Nancy Dow Kathrine Alexander Gulf Coast State College Panhandle Area Educational Consortium 5230 West Highway 98 753 West Boulevard Panama City, Florida 32401 Chipley, Florida 32428 850-769-1551 877-873-7232 www.gulfcoast.edu Pre-test Breaks Explanation of Q & A boards Asking questions Our approach to the standards & to this lesson Florida Next Generation Sunshine State Standards • SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. (MODERATE) Example of a chemical formula : C6H12O6 Example of a molecular structure: Item Specs • Benchmark Clarifications Content Limits BENCHMARK SC.912.L.18.1 Students will identify and/or describe the basic molecular structure of carbohydrates, lipids, proteins, and/or nucleic acids. Students will describe the primary functions of carbohydrates, lipids, proteins, and/or nucleic acids in organisms. Items will not refer to intermolecular forces found in the four types of macromolecules. Items will not assess hydrolysis and dehydration synthesis. Bell ringer We really are what we eat! How does that stuff get to be part of what we are? Elements of Life • 96% of living organisms is made of: carbon (C) oxygen (O) hydrogen (H) nitrogen (N) 3 Molecules of Life • Put C, H, O, N together in different ways to build living organisms • What are bodies made of? – carbohydrates • sugars & starches – proteins – fats (lipids) – nucleic acids • DNA, RNA 4 Don’t forget water • Water – 65% of your body is H2O – water is inorganic • doesn’t contain carbon • Rest of you is made of carbon molecules – organic molecules • • • • carbohydrates proteins fats nucleic acids 5 Carbon atoms have unique bonding properties. • Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. • Carbon-based molecules have three general types of structures. – straight chain – branched chain – ring 6 • Many carbon-based molecules are made of many small subunits bonded together. – Monomers are the individual subunits. – Polymers are made of many monomers. 7 Four main types of carbon-based molecules are found in living things. • Carbohydrates are made of carbon, hydrogen, and oxygen. 8 Four main types of carbon-based molecules are found in living things. • Carbohydrates are made of carbon, hydrogen, and oxygen. – Carbohydrates include sugars and starches. – Monosaccharides are simple sugars.(monomer) – Polysaccharides include starches, cellulose, and glycogen. (polymer) 9 • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. Polymer (cellulose) monomer Cellulose is a polymer of glucose monomers that has a straight, rigid structure 10 Monomers and Polymers • Monomers combine to form Polymers through the process of Dehydration Synthesis. Let’s make some polymers!!!! • …and break them apart to form monomers again. Use the patterns of glucose molecules to build a model of dehydration synthesis. Remember to keep H’s and OH’s that you may remove in order to show that water is also a product of this reaction. The reverse process of Dehydration Synthesis is Hydrolysis. Dehydration – loose water Hydro – water Synthesis – to build or make Lysis – to burst or break What do you need to do to your model to show hydrolysis? • Lipids are nonpolar molecules that include fats, oils, and cholesterol. – Many contain carbon chains called fatty acids. – Fats and oils contain fatty acids bonded to glycerol. Triglyceride 11 • Lipids have several different functions. – broken down as a source of energy – make up cell membranes – used to make hormones 12 • Proteins are polymers of amino acid monomers. – Twenty different amino acids are used to build proteins in organisms. 16 • Proteins are polymers of amino acid monomers. – Twenty different amino acids are used to build proteins in organisms. – Amino acids differ in side groups, or R groups. – Amino acids are linked by peptide bonds. 17 • Proteins differ in the number and order of amino acids. – Amino acids interact to give a protein its shape. Hemoglobin hydrogen bond – Incorrect amino acids change a protein’s structure and function. 18 Hemoglobin in red blood cells transports oxygen. The structure of hemoglobin depends on hydrogen bonds between specific amino acids. Just one amino acid change causes red blood cells to have the curved shape characteristic of sickle cell anemia. (colored SEM; magnification 3500 X) Functions of proteins • Functions—many, including enzymes, oxygen transport, and muscle movement • Nucleic acids are polymers of monomers called nucleotides. 20 • Nucleic acids are polymers of monomers called nucleotides. – Nucleotides are made of a sugar, phosphate group, and a nitrogen base. A phosphate group nitrogen-containing molecule, called a base deoxyribose (sugar) 21 • Nucleic acids are polymers of monomers called nucleotides. – Nucleotides are made of a sugar, phosphate group, and a nitrogen base. DNA – DNA stores genetic information. – RNA transfers genetic RNA information. 22 DNA vs. RNA • Pentose sugars – 5 carbon sugars DNA vs. RNA: Shape • Double Helix Single strand DNA vs. RNA: Nitrogenous Bases • Macromolecule Matching • Complete macromolecule chart • Complete Compare/Contrast Tests for Organic Compounds Lab • Assign different portion of procedures to each group. • Experiment • Compile Data. • Analyze Data. SC.912.L.18.11* Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors, such as pH and temperature, and their effect on enzyme activity. (MODERATE) Item Specs • BENCHMARK SC.912.L.18.1 Benchmark Clarifications Students will explain how enzymes speed up the rate of a biochemical reaction by lowering the reaction’s activation energy. Students will identify and/or describe the effect of environmental factors on enzyme activity. Content Limits Items referring to the role of enzymes as catalysts will use a biological context and not require knowledge of specific enzymes. Items referring to the factors that affect enzyme activity are limited to concentration, pH, and temperature. Items will not require specific knowledge of how an enzyme reacts at a certain pH or temperature. Items will not assess the enzyme-substrate complex. Enzymes Enzymes are proteins that have a particular shape and structure. Site of Activation Characteristics of enzymes • Enzymes are specific – Lock and Key Model • Enzymes are biological catalysts – they speed up chemical reactions without being used up. • Enzymes work at optimum temperatures and pH. What do you think is the optimum temperature for enzymes in the human body? Characteristics of enzymes • Enzymes can be denatured – destroyed. 1. Increase the temperature or 2. Change the pH Cooling or Freezing will slow the enzyme down, but will not denature it. Enzymes – Are a type of protein that act as catalysts, speeding up chemical reactions 1 Active site is available for a molecule of substrate, the reactant on which the enzyme acts. Substrate (lactose) 2 Substrate binds to enzyme. Enzyme Enzyme-substrate complex Glucose OH Enzyme (lactase) H2O galactose H O Enzyme-product complex 4 Products are released. Figure 5.16 3 Substrate is converted to products. Rate of Reaction • Rate of enzyme reactions are based on random collision of molecules – enzyme and substrate molecules. – What effect would substrate concentration have on the rate of reaction? – What happens to the rate of reaction as the substrate molecules are catalyzed? – What happens to the enzyme molecules? Let’s watch some enzyme reactions. 2H2O2 H2O + O2 Effect of Enzyme on Reaction Rate Reactants Free energy Amount of energy released Energy Products Progress of the reaction (a) Exothermic reaction: energy released Free energy Products Energy Reactants Progress of the reaction (b) Endothermic reaction: energy required Enzyme Catalysis Lab • Assign different portion of procedures to each group. • Experiment • Compile Data. • Analyze Data. Follow up • • • • Q/A Board Problem solving issues in class Demo Toothpick-ase Lab Highlight Lactase Lab Post Test