Electronegativity
advertisement
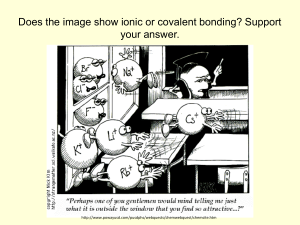
Electronegativity • In a covalent bond between identical atoms eg. H2 and Cl2 the electrons are shared equally between the two atoms • In covalent bonds between different atoms eg. HCl and CO2 one of the atoms attracts the electrons more than the other • In HCl it is found the electrons are more attracted to the chlorine atom • As the electrons spend more time nearer the Chlorine atom the chlorine atom gets a slight negative charge and the hydrogen atom gets a slight positive charge • The small amount of charge is indicated by the Greek symbol “delta” δ • The ability of an atom to attract electrons to itself in a covalent bond is called its Electronegativity • Electronegativity is the relative attraction that an atom in a molecule has for the shared pair of electrons in a covalent bond • Each atom has a certain “pulling power” on electrons • Linus Pauling an American chemist found that Fluorine has four times the “pulling power” of calcium • Flourine has an electronegativity value of 4.0 Calcium has an electronegativity of 1.0 all other elements have electronegativity values relative to these A copy of this table is in your mathematical tables • Elements with low electronegativity values are said to be Electropositive Polar covalent bonds • Because molecules such as HCl contain atoms with different electronegativity values and each atom has a slight charge the molecule is said to be a polar molecule Remember • There are some molecules that have polar bonds but are not polar molecules • This happens when the centre of gravity of the partial negative charges coincides with the partial positive charges Uses of electronegativity Values 1. We can use our knowledge of electronegativity values and the shapes of molecules to predict whether covalent bonds are polar or non polar Significance of polarity in molecules • If water were not polar it would be a gas at room temperature and life could not exist! • Water is known to be an excellent solvent this is because most ionic and polar covalent substances will dissolve in water as there is an attraction between the charges in the water molecules and the charges in the ionic and covalent molecules (detailed explanation p62) Like dissolves like • Polar and ionic substances will dissolve in polar liquids such as water (HCL, NH3) • Non polar substances such as methane, oil and most plastics will not dissolve in water but will dissolve in non polar liquids such as tetrachloroethene or hexane • Dry cleaners use tetrachloroethene to dissolve oily stains from clothes The purple iodine crystals below will not dissolve in water but will dissolve in carbon tetrachloride this is because iodine is non polar 2. We can use electronegativity values to predict whether bonds are ionic or covalent An electronegativity difference greater than 1.7 indicates ionic bonding An electronegativity difference of less than 1.7 indicates polar covalent bonding An electronegativity difference of 0 indicates a pure covalent bond NB !!! A few exceptions • • • • Lithium hydride LiH Sodium Hydride NaH Potassium Hydride KH Calcium Hydride CaH2 • Are all ionic and disobey the “rule of thumb” Try the following Questions • P68 s,t,u 5.2 5.3