Periodic Table
advertisement
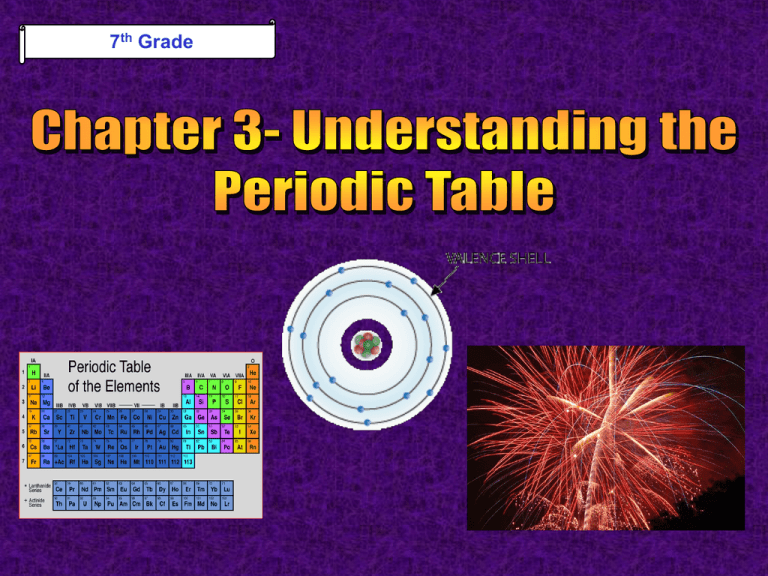
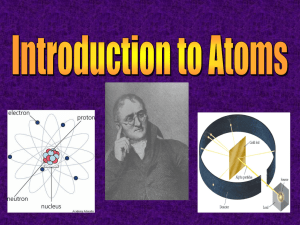
7th Grade 7th Grade Science PISD PowerPoint Lessons Developed By Ryan Gross, Park Crest Middle School Edited By Kenn Heydrick, Coordinator of Science & Health Chapter 3 - Understanding the Periodic Table Section 1: Electrons and the Periodic Table Section 2: Grouping the Elements © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Why Do I Need the Periodic Table? What Do You Think? What does your outer shell of clothing say to other people about you and who you are? © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Electrons & the Periodic Table Electrons in all atoms are arranged around the nucleus in regions called energy levels Energy Levels © Fall 2005, Pflugerville ISD, 8th Grade •The largest atoms have as many as seven energy levels Unit A: Chapter 3 Electrons & the Periodic Table The outermost energy level is called the valence shell Energy Levels © Fall 2005, Pflugerville ISD, 8th Grade The electrons in the valence shell are called valence electrons Unit A: Chapter 3 Electrons & the Periodic Table Elements are grouped because they have similar properties In some groups, the elements all have the same number of valence electrons in their atoms © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Electrons & the Periodic Table In atoms of elements in Groups 1 & 2, the number of valence electrons matches the group number © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Electrons & the Periodic Table In atoms of elements in Groups 13-18, the number of valence electrons is 10 fewer than the group number © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Electrons & the Periodic Table Atoms of elements in Groups 3-12, the Transition Metals, do not follow a general rule •In addition, helium atoms only have 2 valence electrons © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Why Do I Need the Periodic Table? What Do You Think? What similarities exist between you and the other members of your family? How about between you and your classmates? © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Grouping the Elements - Group 1 Group 1: Alkali Metals1 Valence Electron © Fall 2005, Pflugerville ISD, 8th Grade All metals except Hydrogen, the Group 1 elements, are the most reactive. Unit A: Chapter 3 Grouping the Elements - Group 1 Group 1: Alkali Metals1 Valence Electron © Fall 2005, Pflugerville ISD, 8th Grade This means that the atoms of these elements are not stable and will lend valence electrons to other atoms. Unit A: Chapter 3 Grouping the Elements - Group 1 Group 1: Alkali Metals1 Valence Electron These elements are never found uncombined in nature. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Grouping the Elements - Group 1 Alkali Metals Compounds formed from Alkali metals have many uses for humans •NaCl, or Sodium Chloride, is table salt that is used to season your food •Potassium compounds are found in bananas © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Grouping the Elements - Group 2 Group 2: Alkaline-Earth Metals- 2 Valence Electrons Alkaline-Earth metals are very reactive, but not as reactive as Alkali metals. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Grouping the Elements - Group 2 Group 2: Alkaline-Earth Metals- 2 Valence Electrons © Fall 2005, Pflugerville ISD, 8th Grade This is because it is harder for their atoms to lose 2 valence electrons than for the Alkali metals to lose 1 Unit A: Chapter 3 Grouping the Elements - Group 2 Group 2: Alkaline Earth Metals •Magnesium is mixed with other metals to make rims on cars. © Fall 2005, Pflugerville ISD, 8th Grade •Calcium is an important part of the compound that keeps your bones and teeth healthy. Unit A: Chapter 3 Grouping the Elements Groups 3-12 Groups 3-12: Transition Metals Groups 3-12 do not have individual names. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Groups 3-12 Groups 3-12 © Fall 2005, Pflugerville ISD, 8th Grade Groups 3-12 are all grouped together as the Transition Metals. Unit A: Chapter 3 Groups 3-12 Groups 3-12 © Fall 2005, Pflugerville ISD, 8th Grade The Transition Metals are less reactive than Groups 1 & 2 because they don’t lose their valence electrons as easily. Unit A: Chapter 3 Grouping the Elements Silver and Gold are Transition Metals. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Grouping the Elements Transition Metals Iron, Cobalt, and Nickel, all Transition Metals, are the only elements known to produce a magnetic field. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 13 Group 13: Boron Group The most common element from Group 13 is aluminum. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 13 Aluminum was considered more precious than gold or silver until the 1880s, when plentiful electricity made it cheaper. •Aluminum is used to make cans, cars, and airplanes. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 14 Group 14: Carbon Group The nonmetal Carbon, in Group 14, is often found uncombined in nature. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 14 Carbon forms both diamonds and charcoal. Carbon also forms a wide variety of compounds such as proteins, fats, and carbohydrates, all necessary for life on earth. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 15 Group 15: Nitrogen Group Nitrogen, a gas at room temperature, makes up about 78% of the air you breathe. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 15 Group 15: Nitrogen Group Nitrogen from the air is combined with Hydrogen to make fertilizer. Fertilizer helps grow the crops that feed us all. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 16 Oxygen, in Group 16, makes up about 21% of the air you breathe. Oxygen is very reactive, combining with many other elements such as iron and carbon. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 16 Sulfur, another common member of Group 16, is used to make sulfuric acid for car batteries. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 17 Halogens are the very reactive nonmetals in Group 17. They react easily because their atoms only need to gain 1 electron to have a complete set © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 17 Chlorine is a yellow halogen that is used to disinfect water for drinking and swimming © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 18 Noble gases are unreactive nonmetals in Group 18. These elements’ atoms have full outermost energy levels, and cannot react with other elements © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Group 18 Noble gases like neon glow when electrically charged. Argon in a light bulb keeps the filament from burning out. © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Let’s Review! -1- How does the periodic table help you identify the physical properties of elements? © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3 Let’s Review! -2How are elements grouped on the periodic table of elements? © Fall 2005, Pflugerville ISD, 8th Grade Unit A: Chapter 3