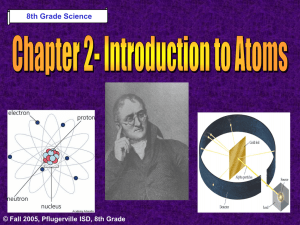
Development of the Atomic Theory Electron Cloud Model The
advertisement

Development of the Atomic Theory What Do You Think? Imagine that you have cut a penny in half. Then, you take one piece and half it again. Will this continue forever, or will you come to a point where no more cutting is possible? © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Democritus was a Greek philosopher who theorized that all matter was made of invisible particles called atoms. Democritus of Abdera, about 460-370 BCE © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory John Dalton 1766-1844 © Fall 2005, Pflugerville ISD, 8th Grade Dalton, a British chemist and teacher, noticed that elements combine in specific proportions to form compounds, and theorized that their atoms combine at the same proportions. Development of the Atomic Theory Thomson’s experiments using a cathode-ray tube showed that smaller particles make up atoms. Joseph John “J.J.” Thomson 1856-1940 © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Rutherford, a former student of Thomson’s from New Zealand, tested his teacher’s theories in his Gold Foil Experiment. Ernest Rutherford 1871- 1937 © Fall 2005, Pflugerville ISD, 8th Grade •He expected his alpha particles to go straight through the foil, and most of them did. Development of the Atomic Theory •But some of the particles were deflected or bounced straight back! Rutherford’s Gold Foil Experiment © Fall 2005, Pflugerville ISD, 8th Grade •This showed that a nucleus with a positive charge makes up the center of an atom. Development of the Atomic Theory Bohr, a Danish scientist who worked with Rutherford, described the motion of electrons around the nucleus. Niels Bohr 1885-1962 © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Bohr’s Atomic Model © Fall 2005, Pflugerville ISD, 8th Grade Bohr said that electrons orbit the nucleus at specific energy levels, and can move from one level to another. Development of the Atomic Theory The current atomic theory states that there are regions inside an atom where electrons are likely to be found. Electron Cloud Model © Fall 2005, Pflugerville ISD, 8th Grade These regions are called electron clouds. The Atom What Do You Think? What is the smallest thing you have ever seen? How does it compare to the size of an atom? © Fall 2005, Pflugerville ISD, 8th Grade Parts of the Atom The nucleus is the small, dense, positively charged center of the atom. © Fall 2005, Pflugerville ISD, 8th Grade Parts of the Atom Protons are positively charged particles in the nucleus. Each proton is equal to 1 atomic mass unit. © Fall 2005, Pflugerville ISD, 8th Grade Parts of the Atom Neutrons are particles in the nucleus that have no charge. Protons and Neutrons are the most massive particles in the atom. © Fall 2005, Pflugerville ISD, 8th Grade Parts of the Atom Electrons are negatively charged particles found in electron clouds outside the nucleus. Super small mass compared to a proton. 1800 electrons = 1 proton © Fall 2005, Pflugerville ISD, 8th Grade Protons and Electrons cancel each other out making atoms neutral. Atomic Number and Isotopes • The number of protons is equal to the number of electrons in atoms. • All atoms of an element have the same number of protons. • The atomic number of an atom is equal to the number of protons in the nucleus. • If atoms have the same number or protons but a different number of neutrons, it is called an isotope. © Fall 2005, Pflugerville ISD, 8th Grade Isotopes • Elements that are isotopes are always the same element. © Fall 2005, Pflugerville ISD, 8th Grade • A different number of neutrons gives it a different mass. Isotope Practice • Helium has 2 protons and 2 neutrons. Its atomic mass is 4. • Carbon has 6 protons and 6 neutrons. Its atomic mass of 12. • Helium-3 (isotope) has an atomic mass of 3. • Carbon-14 (isotope) has an atomic mass of 14. • How many neutrons does it have? • How many neutrons does it have? © Fall 2005, Pflugerville ISD, 8th Grade Atomic Mass • Every element has an atomic mass. • The atomic mass is the sum of the number of protons and neutrons in the nucleus of an atom. © Fall 2005, Pflugerville ISD, 8th Grade • The mass is the average of the masses of all the isotopes of the element. Forces in Atoms • The forces that are always pushing and pulling on atoms are: – – – – Gravitational force Electromagnetic Force Strong Force Weak Force © Fall 2005, Pflugerville ISD, 8th Grade Forces in Atoms • Gravitational Force – Acts between atoms. © Fall 2005, Pflugerville ISD, 8th Grade • Electromagnetic Force – Same charges repel each other. Forces in Atoms • Strong Force – Holds the nucleus together. © Fall 2005, Pflugerville ISD, 8th Grade • Weak Force – In some radioactive elements, this allow some neutrons can turn into protons.