Heat
advertisement

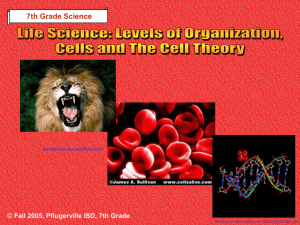
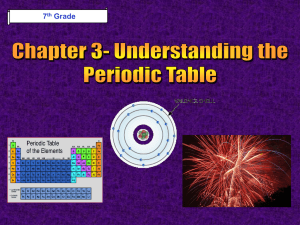
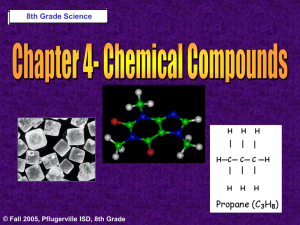
8th Grade Science © Fall 2005, Pflugerville ISD, 8th Grade Chapter 6- Heat Section 1: What is Temperature? Section 2: What is Heat? Section 3: Matter & Heat © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Temperature? What Do You Think? What happens when you use a thermometer to measure temperature? © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Temperature Depends on Kinetic Energy •Kinetic Energy is the energy of motion •The faster the particles of matter move, the more kinetic energy they have © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Temperature? Measuring Temperature •Temperature is a measure of the Average Kinetic Energy of a substance •When you measure temperature, you are measuring the average motion of the atoms © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Temperature? Temperature is expressed by one of 3 scales: © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Temperature? Thermal Expansion- As temperature rises, volume increases © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Temperature? Thermal Expansion •When the metal rails in this picture heated up, they increased in length and volume •Thermal Expansion caused the rails to buckle and bend © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Temperature? Thermal Expansion •The asphalt in the road expands when heated and contracts when cooled •This results in cracks and potholes due to Thermal Expansion © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Heat? What Do You Think? Which is warmer, the tile or the rug on a bathroom floor? © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Heat? Heat- A Transfer of Energy •Heat is the transfer of energy between objects or particles that are at different temperatures © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Heat? Heat- A Transfer of Energy •Energy moves from the warmer object to the cooler object until both are the same temperature •This is known as Thermal Equilibrium © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Heat? Reaching Thermal Equilibrium © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Heat? Energy is Transferred by 3 Methods: © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Heat? •Conduction is the transfer of thermal energy through direct contact •Hold a metal wire in a flame and energy travels from atom to atom until it burns your hand © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Heat? Convection •Convection is the transfer of thermal energy by the movement of a liquid or a gas •Convection is seen as hot material rises and cool material sinks © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 What is Heat? Radiation © Fall 2005, Pflugerville ISD, 8th Grade •Radiation is the transfer of energy through matter or space as EM waves, such as visible light and infrared waves •Radiation is the only type of energy transfer where the objects don’t have to touch Unit A : Chapter 1 : Section 1 Matter & Heat What Do You Think? When you get overheated and start to sweat, your body begins to cool off. Why? © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Matter & Heat States of Matter © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Matter & Heat Ice Crystal •Particles of a solid do not move fast enough to pull apart, so they vibrate in place © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Matter & Heat Melting of Ice •Energy must be transferred to a solid to make it become liquid •The water will remain the same temperature until all of the ice has melted © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Matter & Heat Evaporation of Water •Transfer more energy to the water and its particles move even faster •When the liquid water molecules move fast enough, they become a gas © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Matter & Heat Sweat Cools You Down •When your body gets too hot, you perspire •The liquid sweat uses energy from your body to evaporate water molecules •You get cooler! © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Let’s Review! -1- What is temperature? Explain what happens when you use a thermometer to measure temperature… © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Let’s Review! -2- How is radiation different from conduction and convection? © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Let’s Review! -3- When water evaporates, the air near the water’s surface becomes cooler. Why? © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Harcourt Science: States of Matter BBC: Science BrainPop: States of Matter © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1 Pre-AP Extensions • You should be able to explain Thermal Conductivity and Specific Heat Capacity. • You should know what a Calorimeter is and the difference between a calorie (cal) and a Calorie (C) © Fall 2005, Pflugerville ISD, 8th Grade Unit A : Chapter 1 : Section 1