Chapter 9 Notes
advertisement

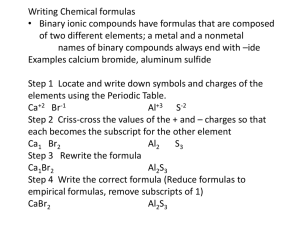
9-1 Notes Naming Ions Monatomic Ions Ionic compounds consist of a positive ion (a metal) bonded to a negative ion (a nonmetal) Ex. KBr Monatomic ions consists of a single atom that has lost or gained electron(s) Ex. Potassium ion lost an electron, while the Bromide ion gained an electron Cations Cations are positively charged ions that occur when atoms lose electrons The # of electrons lost will correspond to the group # on the periodic table Ex. Sodium in group 1 will lose 1 electron Naming Cations – the name is the same as the element the ion is formed from Ex. Sodium forms the sodium cation Identify the cation of the following elements: Ca, Rb, Al, Li, Sr Anions Anions are negatively charged ions that occur when atoms gain electrons Nonmetals in groups 5-7 form anions The charge is determined by subtracting 8 from the group # Ex. Oxygen is in group 6, therefore, 6-8 = -2 Naming Anions – the end of the element’s name changes to –ide Ex. Bromine forms the Bromide anion Identify the anions of the following elements: P, Se, I, F, N Ions of Transition Metals Many of the transition metals form more than 1 cation The charges must be determined from the number of electrons lost Ex. Iron can lose 2 electrons and form Fe2+ or it can lose 3 electrons and form Fe3+ See Table 9.2 p. 255 Ions of Transition Metals There are 2 ways to name these ions 1. Stock System – uses roman numerals to indicate the charge on the ion Ex. Fe2+ is Iron (II) while Fe3+ is Iron (III) 2. Classical name – uses the Latin form of the element and a suffix to indicate the charge The suffix –ous indicates the lower of the 2 charges The suffix –ic indicates the higher of the 2 charges Ex. Fe2+ is the ferrous ion while Fe3+ is the ferric ion A few transition metals only form 1 ion and these are named like all other cations Ex. Silver (Ag+), Zinc (Zn2+) Polyatomic Ions Polyatomic ions are composed of more than 1 atom Ex. Nitrate (NO3-) consists of both nitrogen and oxygen The names of most polyatomic ions end in -ite or –ate The use of –ite or –ate as the ending usually depends on the # of Oxygen atoms in the ion Ex. Nitrate (NO3-) has more oxygen atoms while Nitrite (NO2-) has less oxygen atoms Polyatomic Ions If the formula for a polyatomic ion begins with an H it includes a H+ The charge is the sum of the two ions Ex. Hydrogen carbonate (HCO3-) is the sum of H+ and CO32- Most polyatomic ions are negatively charged The 1 exception is NH4+ which is ammonium See Table 9.3 p. 257 9-2 Notes Naming and Writing Formulas for Ionic Compounds Naming Binary Ionic Compounds A binary compound is composed of 2 elements and can either be ionic or molecular 1st step in naming an ionic compound is making sure it is composed of a monatomic metal cation and a monatomic nonmetal anion When naming, simply place the cation name first followed by the anion name Ex. KBr is Potassium bromide, while Na2O is sodium oxide When the cation is a transition metal with more than 1 charge, you must indicate which ion is being used Ex. Cu2O is Copper (I) oxide, while CuO is Copper (II) oxide Writing Formulas for Binary Ionic Compounds Step 1: Write the symbol of the cation and then the anion. Step 2: Add whatever subscripts are needed to balance the charges Make sure the subscripts are reduced There are two methods to balance charges 1. Find the least common multiple of the charges 2. The crisscross method: the numerical value of the charge of each ion is crossed over and becomes the subscript for the other ion Write the formulas for the following: Iron (III) oxide and Calcium bromide Compounds with Polyatomic Ions Naming ionic compounds with polyatomic ions is the same as binary compounds Write the cation first followed by the anion Name the following: LiCN, AgNO3, (NH4)2C2O4 Compounds with Polyatomic Ions Step 1: Write the symbol of the cation and then the anion. Step 2: Add whatever subscripts are needed to balance the charges Step 3 (if needed): If more than 1 polyatomic ion is needed to balance the charges you must use parentheses Ex. Calcium Nitrate Write the formulas for the following: Lithium carbonate & Aluminum nitrite 9-3 Notes Naming and Writing Formulas for Molecular Compounds Review How do we do the following? Name Ionic Compounds Ex. KBr, Na3PO4, Fe(NO3)3 Write Formulas for Ionic Compounds Ex. Sodium nitride, Ammonium oxide, Copper (II) nitrite Naming Binary Molecular Compounds Binary molecular compounds are composed of molecules, not ions, and consist of 2 nonmetals Due to various ways of sharing electrons the same elements can be used to make different compounds Ex. CO and CO2 How do you name these so you can tell there is a difference? Naming Binary Molecular Compounds Prefixes are used to represent the number of atoms of each element that can be found in a molecular compound The Rules: 1. Confirm the compound is molecular 2. Name the elements in the order listed in the formula 3. Use the appropriate prefix to indicate the # of each element (see table 9.4 p. 269) Exception: Do not use mono- on the 1st element 4. Change the ending of the 2nd element to –ide Name the following: CO, CO2, N2O5, CCl4 Writing Formulas for Molecular Compounds Step 1: Write the correct symbols for the two elements Step 2: Use the prefixes in the name to determine the subscript of each element in the formula Write the formula for the following: Dinitrogen tetraoxide, Dihydrogen monoxide, Phosphorus pentachloride 9-4 Notes Naming and Writing Formulas for Acids and Bases Review How do we do the following? Name Ionic Compounds Ex. (NH4)3PO4, Cr(NO2)3 Write Formulas for Ionic Compounds Ex. Potassium sulfate, Iron (III) oxide Name Molecular Compounds Ex. CO2, CCl4 Write Formulas for Molecular Compounds Ex. Boron Trichloride, Dinitrogen tetrahydride Naming Acids An acid is a compound that contains 1 or more hydrogen atoms and produces H+ when dissolved in water The general form of an acid is HnX n is the subscript indicating how many Hydrogen ions are needed X is the monatomic or polyatomic anion Naming Acids – The Rules 1. If the anion ends in –ide, the acid name will begin with hydro-. The –ide will be replaced by –ic and followed by acid Ex. HCl 2. If the anion ends in –ite, the acid name is the stem of the anion with the suffix –ous and followed by acid Ex. H2SO3 3. If the anion ends in –ate, the acid name is the stem of the anion with the suffix –ic and followed by acid Ex. HNO3 Name the following: HF, HNO2, HMnO4 Writing Formulas for Acids Use the rules for writing the names of acids in reverse to write the formulas for acids Remember – add subscripts to balance the formula Ex. Rule 1 – Hydrobromic acid Ex. Rule 2 – Phosphorous acid Ex. Rule 3 – Sulfuric acid Write formulas for the following: Permanganic acid, Nitrous acid, hydrosulfuric acid Bases A base is an ionic compound that produces hydroxide ions when dissolved in water Naming bases and writing formulas is the same as any other ionic compound Name the following: Ba(OH)2, KOH Write the formulas for the following: Strontium hydroxide, Copper (II) hydroxide